[ Home ] [ Controlled Substances ] [ Opioids ]
TAPENTADOL
|
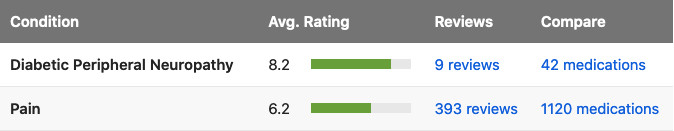
Tapentadol is used for the treatment of moderate to severe pain for both acute (following injury, surgery, etc.) and chronic musculoskeletal pain. It is also specifically indicated for controlling the pain of diabetic neuropathy when around-the-clock opioid medication is required. Extended-release formulations of tapentadol are not indicated for use in the management of acute pain.
Tapentadol

https://www.reddit.com/r/opiates/comments/a3ndp5/love_tapentadol/
Tapentadol is a DEA schedule II opioid medication with a safety profile, duration of action, frequency of dosing, and analgesic effect similar to those of other oral narcotics, although it is more expensive. At low doses, immediate-release tapentadol is better tolerated than oxycodone in terms of gastrointestinal adverse effects, and in the long term, extended-release tapentadol is better tolerated than controlled-release oxycodone. The potential for better gastrointestinal tolerability must be weighed against the higher price.
Safety:
The primary safety concern with tapentadol is respiratory depression.
- It should not be used in patients with severe respiratory illness.
- Older or debilitated patients and those with an underlying respiratory illness should take the lowest effective dose.
- Tapentadol should not be used concurrently with monoamine oxidase inhibitors because of the risk of hemodynamic lability; there is also a theoretical risk of serotonin syndrome in patients taking serotonin reuptake inhibitors.
Concurrent use of extended-release tapentadol and alcohol can be fatal.
The extended-release tablets must not be split, crushed, or broken because rapid absorption may be fatal.
- Tapentadol is a U.S. Food and Drug Administration pregnancy category C drug and should not be used during breastfeeding.
- It has not been studied in children.
- As with all DEA schedule II narcotics, the usual risks of opiate addiction, diversion, and misuse apply.
Tolerability:
Nausea is the most common adverse effect associated with immediate-release tapentadol.
The 50-mg dose has a lower rate of nausea and other gastrointestinal adverse effects (18 to 35 percent) than a 10-mg dose of oxycodone (Oxycontin; 35 to 63 percent).
- At higher doses of tapentadol (75 to 100 mg), rates of nausea are similar to those of oxycodone.
- Extended-release tapentadol has lower rates of nausea and constipation than the controlled-release form of oxycodone.
- Rates of central nervous system adverse effects, such as dizziness, somnolence, headache, and fatigue, are comparable between tapentadol and oxycodone.
Price:
The cost of 50-mg tablets of immediate-release tapentadol, given four times daily for 10 days, is about $132.10. In comparison, oxycodone (10 mg, four times daily for 10 days) costs about $27, and hydromorphone (2 mg, four times daily for 10 days) costs about $31.

https://www.aafp.org/afp/2012/0501/p910.html
Tapentadol is the first US FDA-approved centrally acting analgesic having both u-opioid receptor agonist and noradrenaline (norepinephrine) reuptake inhibition activity with minimal serotonin reuptake inhibition.Tapentadol is indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate, neuropathic pain associated with diabetic peripheral neuropathy (DPN) severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.
 Tapentadol:
Tapentadol:
https://drugs.ncats.io/drug/H8A007M585
Tapentadol Equivalent Doses:
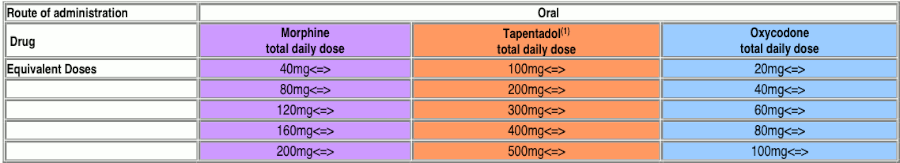


https://www.nhstaysideadtc.scot.nhs.uk/TAPG%20html/pdf%20docs/TAPENTADOL%20DOSE%20CONVERSION%20CHART%20FINAL.pdf
| Duration: An opioid analgesic drug with potency somewhere between tramadol and morphine, and with a similar action to Tramadol. High addiction potential. Potential for respiratory depression in overdose. Should not be combined with depressants or stimulants. NOTE: Insufflated administration is ineffective. | |||
| Route | Onset | Duration | After Effects |
|---|---|---|---|
| Tripsit Factsheets | |||

Tapentadol Basic Information: http://drugs.tripsit.me/tapentadol | |||
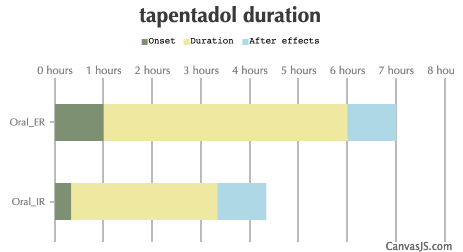
| Oral_IR: | 20-40 minutes | 3-5 hours | 1-12 hours |
| Oral_ER: | 60-120 minutes | 5-8 hours | 1-12 hours |
| |||
| Avoid: All other CNS depressants. | |||
| Effects: Euphoria, Dry Mouth, Mood lift, Itchiness, Relaxant, Constipation, Pupil constriction, Analgesia. | |||
Important Information:
Do not use tapentadol if you have used a MAO inhibitor in the past 14 days. A dangerous drug interaction could occur. MAO inhibitors include isocarboxazid, linezolid, methylene blue injection, phenelzine, rasagiline, selegiline, or tranylcypromine.You should not use this medicine if you have severe breathing problems, or a bowel obstruction called paralytic ileus.
Tapentadol can slow or stop your breathing, especially when you start using this medicine or whenever your dose is changed. Never take this medicine in larger amounts, or for longer than prescribed. Do not crush, break, or open an extended-release tablet. Swallow it whole to avoid exposure to a potentially fatal dose.
Tapentadol may be habit-forming, even at regular doses. Take this medicine exactly as prescribed by your doctor. Never share the medicine with another person. MISUSE OF NARCOTIC PAIN MEDICATION CAN CAUSE ADDICTION, OVERDOSE, OR DEATH, especially in a child or other person using the medicine without a prescription.
Tell your doctor if you are pregnant. Tapentadol may cause life-threatening withdrawal symptoms in a newborn if the mother has taken this medicine during pregnancy.
Fatal side effects can occur if you use this medicine with alcohol, or with other drugs that cause drowsiness or slow your breathing.
Do not drink alcohol. Dangerous side effects or death could occur.
Avoid driving or hazardous activity until you know how this medicine will affect you. Dizziness or drowsiness can cause falls, accidents, or severe injuries.
Interactions:
Drug Interactions (360) Alcohol/Food Interactions (1) Disease Interactions (14)
What other drugs will affect Tapentadol?
Opioid medication can interact with many other drugs and cause dangerous side effects or death. Be sure your doctor knows if you also use:This list is not complete. Other drugs may interact with tapentadol, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible interactions are listed here.
- cold or allergy medicines, bronchodilator asthma/COPD medication, or a diuretic ("water pill");
- medicines for motion sickness, irritable bowel syndrome, or overactive bladder;
- other narcotic medications - opioid pain medicine or prescription cough medicine;
- a sedative like Valium - diazepam, alprazolam, lorazepam, Xanax, Klonopin, Versed, and others;
- drugs that make you sleepy or slow your breathing - a sleeping pill, muscle relaxer, medicine to treat mood disorders or mental illness; or
- drugs that affect serotonin levels in your body - a stimulant, or medicine for depression, Parkinson's disease, migraine headaches, serious infections, or nausea and vomiting.
A total of 360 drugs are known to interact with Tapentadol.
- 180 major drug interactions
- 179 moderate drug interactions
- 1 minor drug interaction
 Tapentadol Drug Information:
Tapentadol Drug Information:
https://www.drugs.com/tapentadol.htmlUser Reviews for Tapentadol:
 User Reviews for Tapentadol (Also known as: Nucynta, Nucynta ER):
User Reviews for Tapentadol (Also known as: Nucynta, Nucynta ER):
https://www.drugs.com/comments/tapentadol/
Tapentadol Drug Interactions:


https://psychonautwiki.org/wiki/Tapentadol
Used for Pain:
- Pain
- Severe chronic pain requiring long-term opioid treatment
- Diabetic complication causing injury to some body nerves
Tapentadol is used to help relieve moderate to severe short-term pain (such as pain from an injury or after surgery). It belongs to a class of drugs known as opioid analgesics. It works in the brain to change how your body feels and responds to pain.
Before Using:
Tell your doctor or pharmacist your medical history, especially of:
- brain disorders (such as seizures, head injury, tumor)
- breathing problems (such as asthma, sleep apnea, chronic obstructive pulmonary disease-COPD)
- gallbladder disease
- kidney disease
- liver disease
- mental/mood disorders (such as confusion, depression, thoughts of suicide)
- personal or family history of a substance use disorder (such as overuse of or addiction to drugs/alcohol)
- stomach/intestinal problems (such as blockage, constipation, diarrhea due to infection, paralytic ileus)
- disease of the pancreas (pancreatitis)
- difficulty urinating (such as due to enlarged prostate)
Precautions:
This drug may make you dizzy or drowsy.
Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness until you can do it safely. Avoid alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).WARNINGS:
Tapentadol has a risk for abuse and addiction, which can lead to overdose and death.
Tapentadol may also cause severe, possibly fatal, breathing problems.
To lower your risk, your doctor should have you take the smallest dose of tapentadol that works, and take it for the shortest possible time.
The risk for severe breathing problems is higher when you start this medication and after a dose increase, or if you take the wrong dose/strength. Taking this medication with alcohol or other drugs that can cause drowsiness or breathing problems may cause very serious side effects, including death. Be sure you know how to take tapentadol and what other drugs you should avoid taking with it. Get medical help right away if any of these very serious side effects occur: slow/shallow breathing, unusual lightheadedness, severe drowsiness/dizziness, difficulty waking up.
User Reviews:
29 Total User Reviews Tapentadol Oral Read Reviews Condition: Cronic Pain (13 Reviews): Effectiveness (3.38) Ease of Use (4.00) Satisfaction (2.92)
 Tapentadol Tablet (Nucynta):
Tapentadol Tablet (Nucynta):
https://www.webmd.com/drugs/2/drug-152562-1209/tapentadol-oral/tapentadol-oral/details
| Side Effects: |
| Get emergency medical help if you have signs of an allergic reaction: hives; chest pain, fast heartbeats, difficult breathing; swelling of your face, lips, tongue, or throat. |
|---|
| |
Nucynta (tapentadol immediate-release oral tablets): https://www.rxlist.com/nucynta-drug/patient-images-side-effects.htm |
| Opioid medicine can slow or stop your breathing, and death may occur. A person caring for you should seek emergency medical attention if you have slow breathing with long pauses, blue colored lips, or if you are hard to wake up. |
Call your doctor at once if you have:
|
| Seek medical attention right away if you have symptoms of serotonin syndrome, such as: agitation, hallucinations, fever, sweating, shivering, fast heart rate, muscle stiffness, twitching, loss of coordination, nausea, vomiting, or diarrhea. |
| Serious side effects may be more likely in older adults and those who are overweight, malnourished, or debilitated. |
| Long-term use of opioid medication may affect fertility (ability to have children) in men or women. It is not known whether opioid effects on fertility are permanent. |
Common side effects may include
|
| This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
Dosage:
Tapentadol is typically started at a low dose and gradually increased until pain is under control. Dosage should be individualized based on pain severity, therapy response, prior treatment experience and risk factors of addiction, abuse and misuse.
- Immediate-release tapentadol tablets are typically prescribed with an initial dosage of 50 to 100 mg taken orally every four to six hours as needed for pain relief. Additional doses may be prescribed for the first day of use to achieve optimum pain relief. The maximum dosage for immediate-release tapentadol tablets is 700 mg on the first day and 600 mg on subsequent days.
- Extended-release tapentadol tablets are typically prescribed with an initial dosage of 50 mg taken twice per day. The maximum dosage of extended-release tapentadol tablets is 500 mg per day.
- Dosage should be individualized to provide adequate pain relief yet minimize adverse reactions.
- If changing from immediate-release to extended-release tapentadol, the current daily dosage of immediate-release should be divided into two equal doses of extended-release and taken orally twice per day, 12 hours apart.

https://www.painscale.com/article/tapentadol-medication
| Maximum Dosage: | |
|---|---|
| Prescribers Digital Reference | |

Nucynta - Drug Summary: https://www.pdr.net/drug-summary/Belsomra-suvorexant-3605 | |
| Adults: | 500 mg/day PO for extended-release; 700 mg PO on Day 1, then 600 mg/day PO thereafter for immediate-release. |
| Geriatric: | 500 mg/day PO for extended-release; 700 mg PO on Day 1, then 600 mg/day PO thereafter for immediate-release. |
| Adolescents: | Safety and efficacy have not been established. |
| Children: | Safety and efficacy have not been established. |
| Infants: | Safety and efficacy have not been established. |
| Neonates: | Safety and efficacy have not been established. |
| Black Box Warnings: |
|---|

Tapentadol (Rx): https://reference.medscape.com/drug/nucynta-tapentadol-999202 |
Opioid analgesic risk evaluation and mitigation strategy (REMS)
Addiction, abuse, and misuse
Life-threatening respiratory depression
Accidental exposure
Neonatal opioid withdrawal syndrome
Interaction with alcohol
Interaction with benzodiazepines and other CNS depressants
|
Pediatric:
Appropriate studies have not been performed on the relationship of age to the effects of tapentadol in the pediatric population.Safety and efficacy have not been established.
Geriatric:
Appropriate studies performed to date have not demonstrated geriatric-specific problems that would limit the usefulness of tapentadol in the elderly. However, elderly patients are more likely to have constipation and age-related lung, liver, or kidney problems, which may require caution and an adjustment in the dose for patients receiving tapentadol.Other Medical Problems:
The presence of other medical problems may affect the use of this medicine. Make sure you tell your doctor if you have any other medical problems, especially:
- Adrenal problems
- Alcohol abuse, history of
- Brain tumor, history of
- Breathing or other lung problems (eg, chronic obstructive pulmonary disease or COPD, sleep apnea)
- Depression, history of
- Drug abuse or dependence, history of
- Gallbladder problems
- Head injuries, history of
- Kyphoscoliosis (severe curvature of the spine that can cause breathing problems)
- Weakened physical condition - Use with caution. May increase risk for more serious side effects
- Gallbladder problems
- Hypotension (low blood pressure)
- Pancreatitis (swelling of the pancreas)
- Seizures or epilepsy, history of - Use with caution. May make these conditions worse.
- Kidney disease, severe
- Liver disease, severe - Use is not recommended in patients with these conditions
- Liver disease, moderate - Use with caution. The effects may be increased because of slower removal of the medicine from the body
- Lung or breathing problems (eg, asthma, hypercarbia, respiratory depression), severe
- Stomach or bowel blockage (including paralytic ileus) - Should not be used in patients with these conditions
 Tapentadol (Oral Route) (Nucynta | Nucynta ER):
Tapentadol (Oral Route) (Nucynta | Nucynta ER):
https://www.mayoclinic.org/drugs-supplements/tapentadol-oral-route/description/drg-20072580
Breastfeeding:Summary of Use During Lactation:
Little information is available on the use of tapentadol during breastfeeding.
Because it has opioid agonist activity, an alternate drug is preferred, especially while nursing a newborn or preterm infant. Newborn infants seem to be particularly sensitive to the effects of even small dosages of narcotic analgesics. Monitor infants for excess sedation and respiratory depression. If the baby shows signs of increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness, a physician should be contacted immediately. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breastfeeding is stopped.Effects in Breastfed Infants:
The German manufacturer of tapentadol reports that they received 4 spontaneous reports of infant exposure to tapentadol in breastmilk, with no adverse reactions noted. Neither the age of the infants nor the extent of breastfeeding were reported. The dosage was known in only one of the mothers, which was 100 mg twice daily given rectally.Alternate Drugs to Consider:
- Acetaminophen
- Butorphanol
- Hydromorphone
- Ibuprofen
- Morphine
 Tapentadol Drug Levels and Effects:
Tapentadol Drug Levels and Effects:
https://www.ncbi.nlm.nih.gov/books/NBK537996/
What is the most important information I should know about tapentadol?
MISUSE OF OPIOID MEDICINE CAN CAUSE ADDICTION, OVERDOSE, OR DEATH.
- Keep the medication in a place where others cannot get to it.
- Taking opioid medicine during pregnancy may cause life-threatening withdrawal symptoms in the newborn.
- Fatal side effects can occur if you use opioid medicine with alcohol, or with other drugs that cause drowsiness or slow your breathing.
 Tapentadol (Brand: Nucynta, Nucynta ER):
Tapentadol (Brand: Nucynta, Nucynta ER):
https://www.uofmhealth.org/health-library/d07453a1
IMPORTANT WARNING:
Tapentadol may be habit forming, especially with prolonged use. Take tapentadol exactly as directed. Do not take more of it, take it more often, or take it in a different way than directed by your doctor. While taking tapentadol, discuss with your healthcare provider your pain treatment goals, length of treatment, and other ways to manage your pain. Tell your doctor if you or anyone in your family drinks or has ever drunk large amounts of alcohol, uses or has ever used street drugs, or has overused prescription medications, or if you have or have ever had depression or another mental illness. There is a greater risk that you will overuse tapentadol if you have or have ever had any of these conditions. Talk to your healthcare provider immediately and ask for guidance if you think that you have an opioid addiction or call the U.S. Substance Abuse and Mental Health Services Administration (SAMHSA) National Helpline at 1-800-662-HELP.Tapentadol may cause serious or life-threatening breathing problems, especially during the first 24 to 72 hours of your treatment and any time your dose is increased. Your doctor will adjust your dose to control your pain and decrease the risk that you will experience serious breathing problems. Tell your doctor if you have or have ever had slowed breathing or asthma. Your doctor will probably tell you not to take tapentadol. Also tell your doctor if you have or have ever had lung disease such as chronic obstructive pulmonary disease (COPD; a group of lung diseases that includes chronic bronchitis and emphysema), a head injury, or any condition that increases the amount of pressure in your brain. The risk that you will develop breathing problems may be higher if you are an older adult or are weakened or malnourished due to disease. If you experience any of the following symptoms, call your doctor immediately or get emergency medical treatment: slowed breathing, long pauses between breaths, or shortness of breath.
Taking certain other medications during your treatment with tapentadol may increase the risk that you will experience breathing problems or other serious, life threatening breathing problems, sedation, or coma. Tell your doctor if you are taking or plan to take any of the following medications: benzodiazepines such as alprazolam (Xanax), diazepam (Diastat, Valium), estazolam, flurazepam, lorazepam (Ativan), and triazolam (Halcion); other narcotic pain medications; medications for mental illness or nausea; muscle relaxants; sedatives; sleeping pills; or tranquilizers. Your doctor may need to change the dosages of your medications and will monitor you carefully. If you take tapentadol with any of these medications and you develop any of the following symptoms, call your doctor immediately or seek emergency medical care: unusual dizziness, lightheadedness, extreme sleepiness, slowed or difficult breathing, or unresponsiveness. Be sure that your caregiver or family members know which symptoms may be serious so they can call the doctor or emergency medical care if you are unable to seek treatment on your own.
Drinking alcohol, taking prescription or nonprescription medications that contain alcohol, or using street drugs during your treatment with tapentadol increases the risk that you will experience serious, life-threatening side effects. Do not drink alcohol, take prescription or nonprescription medications that contain alcohol, or use street drugs during your treatment with tapentadol.
Do not allow anyone else to take your medication. Tapentadol may harm or cause death to other people who take your medication, especially children. Keep tapentadol in a safe place so that no one else can take it accidentally or on purpose. Be especially careful to keep tapentadol out of the reach of children. Keep track of how many tablets or extended-release tablets are left so you will know if any medication is missing. Flush any tablets or extended-release tablets that are outdated or no longer needed down the toilet so that others will not take them.
If you are taking the extended-release tablets, swallow them whole; do not chew, break, divide, crush, or dissolve them. If you swallow broken, chewed, crushed, or dissolved extended-release tablets, you may receive too much tapentadol at once instead of slowly over 12 hours. This may cause serious problems, including overdose and death.
Tell your doctor if you are pregnant or plan to become pregnant. If you take tapentadol regularly during your pregnancy, your baby may experience life-threatening withdrawal symptoms after birth. Tell your baby's doctor right away if your baby experiences any of the following symptoms: irritability, hyperactivity, abnormal sleep, high-pitched cry, uncontrollable shaking of a part of the body, vomiting, diarrhea, or failure to gain weight.
Talk to your doctor about the risks of taking tapentadol.
Your doctor or pharmacist will give you the manufacturer's patient information sheet (Medication Guide) when you begin your treatment with tapentadol and each time you fill your prescription. Read the information carefully and ask your doctor or pharmacist if you have any questions. You can also visit the Food and Drug Administration (FDA) website (http://www.fda.gov/Drugs/DrugSafety/ucm085729.htm) or the manufacturer's website to obtain the Medication Guide.

https://www.reddit.com/search/?q=tapentadol

https://erowid.org/experiences/subs/exp_Tapentadol.shtml

https://www.everydayhealth.com/drugs/nucynta/reviews


https://www.who.int/medicines/areas/quality_safety/TAPENTADOL_IFPMA_Comments.pdf


https://www.who.int/medicines/areas/quality_safety/5.2ExpertreviewTapentadolpre-review.pdf


https://www.nucynta.com/assets/pdf/2019_Nucynta_ER_HCP_ISI.pdf


https://www.nucynta.com/assets/pdf/2019_NER_PI.pdf


https://www.nucynta.com/assets/pdf/2019_Nucynta_HCP_ISI.pdf


https://www.caymanchem.com/msdss/9000620m.pdf
- Opioid analgesic
- It is similar to tramadol in its action but unlike tramadol, it has only weak effects on the reuptake of serotonin and is a significantly more potent opioid with no known active metabolites.
- It is generally regarded as a weak-moderate strength opioid (a category shared by many better-known opioids such as hydrocodone and pethidine).
- The potency of tapentadol is somewhere between that of tramadol and morphine, with an analgesic efficacy comparable to that of oxycodone despite a lower incidence of side effects.
- Analgesia occurs within 32 minutes of oral administration, and lasts for 4 - 6 hours
- Tapentadol was approved by the US FDA in 2008. Australia 2010, UK 2011, India 2011 and 2013 for Extended Release.
- The most commonly reported side effects of tapentadol therapy in clinical trials were nausea, vomiting, dizziness, sleepiness, itchiness, dry mouth, headache, and fatigue.
Tapentadol is used for the treatment of moderate to severe pain for both acute (following injury, surgery, etc.) and chronic musculoskeletal pain. It is also specifically indicated for controlling the pain of diabetic neuropathy when around-the-clock opioid medication is required. Extended-release formulations of tapentadol are not indicated for use in the management of acute pain.
Invented in Germany late 1980s by the same company that invented Tramadol. the team aimed to discover a single molecule that minimized the serotonin activity, had strong opioid receptor agonism and strong norepinephrine reuptake inhibition, and would not require metabolism to be active; the result was tapentadol.
Tapentadol is not a pro-drug and therefore does not rely on metabolism to produce its therapeutic effects; this makes it a useful moderate-potency analgesic option for patients who do not respond adequately to more commonly used opioids due to genetic disposition
After annual sales of $166 million, in 2015, Johnson & Johnson sold its rights to market tapentadol in the US to Depomed for $1 billion. It was manufactured by Depomed at a plant located on the island of Puerto Rico that was hit by Hurricane Maria in 2017 causing a major shortage in the drug's availability. In January 2018 Depomed sold off the manufacturing of the drug and licensed it to Collegium Pharmaceutical for $10 million up front with an annual royalty payment of a minimum $135 million for the next 4 years. This combination of events has caused additional short supply of the drug leaving patients who depend on it to seek alternative treatments.
Tapentadol is 'Third-Tier' Drug for Diabetic Neuropathic Pain - The opioid analgesic tapentadol (Nucynta, Janssen Pharmaceuticals) is approved for use in patients with painful diabetic peripheral neuropathy in the United States, but it is expensive, and most ... Tuesday June 03, 2014 - medscape.com Slidell man arrested, accused of dealing tapentadol pills - A Slidell man has been arrested and accused of dealing Tapentadol pills across St. Tammany Parish. According to the St. Tammany Parish Sheriff's Office, Wilfried Cousin, 31, of Slidell, was arrested ... Tapentadol drug abuse high in Kovai but can’t register cases: Cops - “Abuse of prescription drugs is not new, but the rise in use of opiod drugs has risen because we intensified surveillance against smuggling of ganja and other contraband items,” the police said. FDA Approves NUCYNTA® ER (tapentadol) Extended-Release Oral Tablets for the Management of Neuropathic Pain Associated with Diabetic Peripheral Neuropathy - FDA Approves NUCYNTA ® ER (tapentadol) Extended-Release Oral Tablets for the Management of Neuropathic Pain Associated with Diabetic Peripheral Neuropathy Clinical Trial Data Demonstrate Efficacy and ... FDA Approves Tapentadol for Chronic Pain - August 26, 2011 (UPDATED September 13, 2011) — The US Food and Drug Administration (FDA) has approved tapentadol extended release (Nucynta, Janssen Pharmaceuticals) for moderate to severe chronic pain ... 'Tablet Peddlers’ find new modes to smuggle tapentadol to Trichy - Trichy: With Trichy police and Tamil Nadu drug administration department intensifying the vigil against tapentadol drug abuse, the most popular form of addiction among youths involving painkiller ... FDA Approves Tapentadol Immediate-Release Tablets for Relief of Moderate to Severe Acute Pain - RARITAN, N.J., Nov. 21 Millions of Americans with moderate to severe acute pain and their health-care providers will soon have a new treatment option. Today, Johnson & Johnson Pharmaceutical Research ... Supplemental New Drug Application Submitted to FDA for NUCYNTA® ER (Tapentadol) Extended-Release Tablets for Diabetic Peripheral - TITUSVILLE, N.J., Oct. 31, 2011 /PRNewswire via COMTEX/ -- Johnson & Johnson Pharmaceutical Research & Development, L.L.C. (J&JPRD) announced today that it has submitted a supplemental New Drug ... NUCYNTA® ER (Tapentadol Extended-Release Tablets) Receives FDA Approval for the Management of Moderate to Severe Chronic Pain - RARITAN, N.J., Aug. 26, 2011 /PRNewswire via COMTEX/ -- Janssen Pharmaceuticals, Inc. today announced the U.S. Food and Drug Administration (FDA) has approved NUCYNTA® ER, an oral analgesic taken ... Tapster (Tapentadol) Drug Price and Information - The Price section compares costs of the same generic drugs across brands and is purely for information purpose. Medindia neither buys nor sells drugs. Tapentadol (Tapster) is an analgesic, prescribed ... FDA Approves NUCYNTA® ER (tapentadol) Extended-Release Oral Tablets for the Management of Neuropathic Pain Associated with Diabetic Peripheral Neuropathy - Raritan, N.J., August 29, 2012 /PRNewswire/ — Janssen Pharmaceuticals, Inc. today announced the U.S. Food and Drug Administration (FDA) has approved the supplemental New Drug Application (sNDA) for ...
| ||
| Opioids | Link to this page |