[ Home ] [ Controlled Substances ] [ Depressants ]
CLOBAZAM
|
Clobazam has been used to treat anxiety and epilepsy since 1970s. In the US clobazam was approved for marketing in October of 2011 for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome. It is also approved for adjunctive therapy for epilepsy in patients who have not responded to first-line drugs and in children who are refractory to first-line drugs. The mechanism of action for clobazam is not fully understood but is thought to involve what is known as potentiation of GABAergic neurotransmission resulting from binding at a benzodiazepine site at the GABA(A) receptor. Possible side effects: constipation, fever, drowsiness, sedation, ataxia, aggressive behavior, lethargy, drooling, and irritability. Other side effects include: urinary tract infection, pneumonia, cough, dysphagia, dysarthria, bronchitis, insomnia, fatigue, decreased appetite, and increased appetite.
 Clobazam:
Clobazam:
https://drugs.ncats.io/drug/2MRO291B4U
Clobazam is a benzodiazepine which belongs to the same family of drugs that includes lorazepam (Ativan), diazepam (Valium), midazolam (Versed) and clonazepam (Klonopin). Clobazam is different from the other benzodiazepines because it is utilized for the long-term treatment of epilepsy due to its effectiveness and relatively low tendency to produce sedation.
The medication is indicated for use in patients as an add- on medication for seizures associated with Lennox-Gastaut syndrome. Patients must be 2 years of age or older.

https://www.epilepsy.com/medications/clobazam/advanced
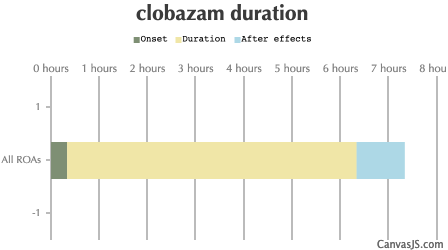
| Duration: A long acting benzodiazepine which focuses primarily on anxiolytic and anticonvulsant properties. It is used medically to treat epilepsy, and this along with its relatively many negative side-effects and contraindications in comparison with other benzodiazepines means it isn't frequently used recreationally. NOTE: 20mg of Clobazam is approximately equal to 10mg Diazepam. | |||
| Route | Onset | Duration | After Effects |
|---|---|---|---|
| Tripsit Factsheets | |||

Clobazam Basic Information: http://drugs.tripsit.me/clobazam | |||
| All ROAs: | 20-40 minutes | 6-12 hours | 1-12 hours |
| |||
| Avoid: All other CNS depressants. | |||
Aliases:
| |||
| Summary: A long acting half life benzo that focues on Anxiolytic and/or Anticonvulsant. | |||
| Effects: Anxiolytic, Sedative, Muscle Relaxant, Amnesia, Dystaxia. | |||
Breastfeeding:
Summary of Use During Lactation:
Limited information indicates that maternal doses of clobazam up to 30 mg daily produce low levels in milk.Short-term use would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months.
During long-term administration, monitor the infant for possible sedation and poor sucking and weight gain.Alternate Drugs to Consider:
- (Seizure Disorder) Carbamazepine
- Divalproex
- Gabapentin
- Lamotrigine
- Oxcarbazepine
- Phenytoin
- Valproic Acid
 Clobazam Drug Levels and Effects:
Clobazam Drug Levels and Effects:
https://www.ncbi.nlm.nih.gov/books/NBK501854/

https://erowid.org/experiences/subs/exp_Clobazam.shtml


https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203993s005lbl.pdf


https://www.medicines.org.uk/emc/files/pil.5029.pdf
Important Information:
Some people have thoughts about suicide while taking clobazam. Stay alert to changes in your mood or symptoms. Report any new or worsening symptoms to your doctor.Fatal side effects can occur if you use clobazam with opioid medication, alcohol, or other drugs that cause drowsiness or slow your breathing.
Do not stop using clobazam suddenly, or you could have increased seizures or unpleasant withdrawal symptoms.This medication may impair your thinking or reactions. Be careful if you drive or do anything that requires you to be alert.
Do not drink alcohol. Dangerous side effects could occur.
Interactions:
Drug Interactions (471) Alcohol/Food Interactions (1) Disease Interactions (3)
What other drugs will affect Clobazam?
Taking clobazam with other drugs that make you sleepy or slow your breathing can cause dangerous side effects or death.
Ask your doctor before using opioid medication, a sleeping pill, a muscle relaxer, or medicine for anxiety or seizures. Other drugs may affect clobazam, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all your current medicines and any medicine you start or stop using.A total of 471 drugs are known to interact with Clobazam.
- 29 major drug interactions
- 408 moderate drug interactions
- 34 minor drug interactions
 Clobazam Drug Information:
Clobazam Drug Information:
https://www.drugs.com/mtm/clobazam.html
 Drugs.com Frisium Leaflet:
Drugs.com Frisium Leaflet:
https://www.drugs.com/uk/clobazam-10mg-tablets-leaflet.html


https://medsafe.govt.nz/profs/Datasheet/f/Frisiumtab.pdf
IMPORTANT WARNING:
Clobazam may increase the risk of serious or life-threatening breathing problems, sedation, or coma if used along with certain medications. Tell your doctor if you are taking or plan to take: antidepressants; medications for anxiety, mental illness, and seizures; sedatives; sleeping pills; opioids such as codeine, fentanyl (Duragesic, Subsys), morphine (Astramorph, Kadian), or oxycodone (in Percocet, in Roxicet, others); or tranquilizers. Your doctor may need to change the dosages of your medications and will monitor you carefully. If you take clobazam with any of these medications and you develop any of the following symptoms, call your doctor immediately or seek emergency medical care immediately: unusual dizziness, lightheadedness, extreme sleepiness, slowed or difficult breathing, or unresponsiveness. Be sure that your caregiver or family members know which symptoms may be serious so they can call the doctor or emergency medical care if you are unable to seek treatment on your own. Drinking alcohol or using street drugs during your treatment with clobazam also increases the risk that you will experience these serious, life-threatening side effects. Do not drink alcohol or use street drugs during your treatment.
 Medlineplus Clobazam:
Medlineplus Clobazam:
https://medlineplus.gov/druginfo/meds/a612008.html
| Maximum Dosage: | |
|---|---|
| Prescribers Digital Reference | |

Onfi (clobazam) - Drug Summary: https://www.pdr.net/drug-summary/Onfi-clobazam-295 | |
| Adults: | 40 mg/day PO. |
| Geriatric: | 40 mg/day PO. |
| Adolescents: | Weighing more than 30 kg: 40 mg/day PO. |
| Adolescents: | Weighing 30 kg or less: 20 mg/day PO. |
| Children: | 2 to 12 years weighing more than 30 kg: 40 mg/day PO. |
| Children: | 2 to 12 years weighing 30 kg or less: 20 mg/day PO. |
| Children: | 1 year: Safety and efficacy have not been established; however, doses up to 2 mg/kg/day PO have been recommended for Dravet syndrome. |
| Infants: | Safety and efficacy have not been established; however, doses up to 2 mg/kg/day PO have been recommended for Dravet syndrome. |
| Neonates: | Safety and efficacy have not been established. |
Pediatric:
Appropriate studies have not been performed on the relationship of age to the effects of clobazam in children younger than 2 years of age.However, safety and efficacy in children with panic disorder have not been established.
Geriatric:
Appropriate studies performed to date have not demonstrated geriatric-specific problems that would limit the usefulness of clobazam in the elderly. However, elderly patients are more likely to have age-related liver problems, which may require caution and an adjustment in the dose for patients receiving clobazam.Other Interactions:
- Ethanol
Other Medical Problems:
Make sure you tell your doctor if you have any other medical problems, especially:
- Alcohol abuse, or history of
- Depression, history of
- Drug abuse or dependence, or history of
- Lung or breathing problems (eg, respiratory depression)
- Mood or behavior disorder, history of - Use with caution. May make these conditions worse
- Liver disease, mild to moderate - Use with caution. The effects may be increased because of slower removal of the medicine from the body
 Clobazam (Oral Route) (Onfi):
Clobazam (Oral Route) (Onfi):
https://www.mayoclinic.org/drugs-supplements/clobazam-oral-route/description/drg-20075333
| Side Effects: |
| Get emergency medical help if you have signs of an allergic reaction (hives, difficult breathing, swelling in your face or throat) or a severe skin reaction (fever, sore throat, burning eyes, skin pain, red or purple skin rash with blistering and peeling). |
|---|
| RxList |
Onfi (clobazam tablets and oral suspension): https://www.rxlist.com/onfi-drug/patient-images-side-effects.htm |
| Report any new or worsening symptoms to your doctor, such as: mood or behavior changes, anxiety, panic attacks, trouble sleeping, or if you feel impulsive, irritable, agitated, hostile, aggressive, restless, hyperactive (mentally or physically), more depressed, or have thoughts about suicide or hurting yourself. |
Call your doctor at once if you have:
|
| The sedative effects of clobazam may last longer in older adults. Accidental falls are common in elderly patients who take benzodiazepines. Use caution to avoid falling or accidental injury while you are taking clobazam. |
Common side effects may include:
|
| This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
| Black Box Warnings: |
|---|

ONFI, Sympazan (clobazam) (Rx): https://reference.medscape.com/drug/onfi-sympazan-clobazam-999696 |
Concomitant use of benzodiazepines and opioids may result in profound respiratory depression, coma, and death; administer concomitantly when there are no alternative options; limit dosages and durations to minimum required; monitor for signs and symptoms of respiratory depression and sedationAddiction, abuse, and misuse
|
Liver:
Therapy with clobazam has not been associated with serum aminotransferase elevations, and clinically apparent liver injury from clobazam has yet to be reported and must be rare, if it occurs at all.
Clobazam Hepatotoxicity:
Limited data are available on the hepatotoxicity of clobazam. In clinical trials, clobazam was not associated with an increased frequency of serum aminotransferase elevations as compared to placebo treatment, and there were no instances of clinically apparent liver injury. No individual case reports of clobazam hepatotoxicity have been published since its wide spread clinical availability. Liver injury from benzodiazepines is quite rare, but isolated instances of hepatotoxicity have been reported for clorazepate and clonazepam, two other benzodiazepines used predominantly in the therapy of epilepsy. Thus, clinically apparent liver injury due to clobazam must be rare, if it occurs at all.E Likelihood score: E (unlikely cause of clinically apparent liver injury).
 Clobazam Overview:
Clobazam Overview:
https://www.ncbi.nlm.nih.gov/books/NBK548865/

https://www.onfi.com/

https://www.sympazan.com.com/
Clobazam:
- A benzodiazepine
- Used for its anxiolytic effect, and as an adjunctive therapy in epilepsy
- First synthesized in 1966
- Patented in 1968
- First published in 1969
- Marketed in 1970
- Originally marketed as an anxioselective anxiolytic and an anticonvulsant since 1984
The primary drug-development goal was to provide greater anxiolytic, anti-obsessive efficacy with fewer benzodiazepine-related side effects.
- Common side effects include fever, lethargy, sleepiness, drooling, and constipation
Clobazam Rash: New Warning for an Old Drug - On December 3, 2013, the US Food and Drug Administration (FDA) issued a drug safety communication warning of "rare but serious" skin reactions from the antiseizure drug clobazam. [1] These included ... Monday January 27, 2014 - medscape.com Clobaday (20 mg) (Clobazam) Drug Price and Information - The Price section compares costs of the same generic drugs across brands and is purely for information purpose. Medindia neither buys nor sells drugs. Clobazam (Clobaday (20 mg)) is a benzodiazepine ... FDA approves clobazam (Onfi) for epilepsy - (CBS) The drug clobazam (Onfi) has been approved by the FDA for the treatment of a rare but severe form of epilepsy in adults and in children age two or older. "Lennox-Gastaut syndrome is a severe ... FDA Approves Clobazam Add-On for Lennox Syndrome - October 24, 2011 — The US Food and Drug Administration has approved clobazam (Onfi, Lundbeck) as add-on therapy for seizures associated with Lennox-Gastaut syndrome. Clobazam is already available ... Lundbeck says clobazam effective in seizures - COPENHAGEN, Dec 6 (Reuters) - Danish drugmaker H Lundbeck said it got positive results in a Phase 3 trial of its candidate drug clobazam in treating seizures associated with Lennox-Gastaut syndrome. FDA Warns of Serious Drug Reaction Linked to Clobazam, Levetiracetam - Patients prescribed levetiracetam or clobazam should be informed about the risk of DRESS. The Food and Drug Administration (FDA) has issued a safety alert regarding the risk of DRESS (drug reaction ... Lundbeck Reports Positive Phase III Study Results for Clobazam in the Adjunctive Treatment of Seizures Associated with Len - Two highest dosages of clobazam as add-on therapy achieved a robust statistically significant reduction in average weekly rate of drop seizures, compared with placebo 1 77.6 percent of patients who ... FDA Warns of Rare Life-Threatening Reaction to Anti-Seizure Drugs - Two anti-seizure drugs -- levetiracetam (Keppra and others) and clobazam (Onfi, Sympazan) -- can cause a rare but serious hypersensitivity reaction known as drug reaction with eosinophilia and ... Clobaday (10 mg) (Clobazam) Drug Price and Information - Clobazam (Clobaday (10 mg)) is a benzodiazepine derivative, prescribed for seizure disorders. The information provided on this page is intended to serve as a comprehensive resource and should not be a ... Lundbeck says clobazam effective in seizures - Phase 3 clobazam trial gets good results treating seizures Company repeats will file application with FDA by year-end COPENHAGEN, Dec 6 (Reuters) - Danish drugmaker Lundbeck has got positive results ... Lundbeck Reports Positive Phase III Study Results for Clobazam in the Adjunctive Treatment of Seizures Associated with Len - Two highest dosages of clobazam as add-on therapy achieved a robust statistically significant reduction in average weekly rate of drop seizures, compared with placebo 1 77.6 percent of patients who ...
| ||
| Depressants | Link to this page |