[ Home ] [ Glossary ] [ Controlled Substances ]
| Synthetic Cannabinoids: |
 |

Spice / K2, Synthetic Marajuana
Synthetic cannabinoids are not one drug. Hundreds of different synthetic cannabinoid chemicals are manufactured and sold. New ones with unknown health risks become available each year. Synthetic cannabinoids are popular because users often believe they are legal and relatively safe. These chemicals are called cannabinoids because they act on the same brain cell receptors as tetrahydrocannabinol (THC), the main active ingredient in marijuana. However, the hundreds of known synthetic cannabinoid chemicals and THC are different chemicals. In fact, synthetic cannabinoids may affect the brain in different and unpredictable ways compared to marijuana.
Synthetic cannabinoids are used in a variety of ways:
- Sprayed onto plant material and smoked
- Mixed into a liquid and vaped in electronic nicotine delivery devices (such as e-cigarettes)
- Added to herbal tea or to food and swallowed
Many synthetic cannabinoids are illegal:
- The federal government has banned many specific synthetic cannabinoids. Many state and local governments have passed their own laws targeting other synthetic cannabinoids.
- Recent federal and state laws targeting synthetic cannabinoids have banned general categories of ingredients, rather than specific chemicals.
- Makers of synthetic cannabinoids try to get around these laws by creating new products with different ingredients or by labeling them "not for human consumption."

https://www.cdc.gov/nceh/hsb/chemicals/sc/About.html
https://adf.org.au/drug-facts/synthetic-cannabis/
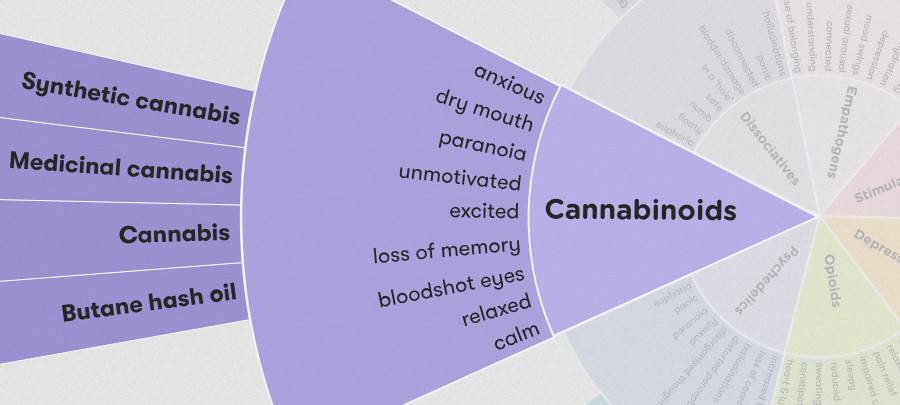
Synthetic cannabinoids (synthetic marijuana, Spice, K2) are various man made chemicals that some people may use as an alternative to marijuana. These seemingly innocent little packages of "fake weed" can cause serious side effects that are very different from those of marijuana.
Synthetic cannabinoid products can be toxic. As a result, people who smoke these products can react with rapid heart rate, vomiting, agitation, confusion, and hallucinations. Some have to get help from emergency medical services or in hospital emergency departments or intensive care units.

https://www.cdc.gov/nceh/hsb/chemicals/sc/default.html

Synthetic cannabinoids are marketed as legal and typically consist of plant material coated by chemicals, which are supposed to mimic THC, the active chemical compound in marijuana. The drugs are marketed as incense, herbal mixtures, or potpourri in order to mask their true purpose. Street names for substances include Spice, K2, Green Giant, Smacked, Wicked X, AK-47, Geeked Up, Ninja, Caution, Red Giant, and Keisha Kole, XXX Ultra, Skunk, Atomic and many more. Users of the synthetic mixtures can never be certain in which ways the drugs will harm them, but users have experienced symptoms that include renal failure, arrested heart rate, high blood pressure, loss of consciousness, violent behavior, nausea, vomiting, tremors, seizures, hallucinations, paranoia, agitation, anxiety, and even death. These effects can be similar to those of phencyclidine, or PCP.

https://www.health.ny.gov/professionals/narcotic/synthetic_cannabinoids/
Synthetic Cannabinoids are chemical compounds that mimic the effects of THC the main active ingredient of cannabis. They bind to the cannabinoid receptors in the brain and were developed to treat pain.
First Generation Synthetic Cannabinoids:
New Generation Synthetic Cannabinoids:
The illicit use of the synthetic cannabinoids throughout the United States, resulting in severe adverse effects, overdoses and deaths. While new synthetic cannabinoids continue to emerge on the illicit market, some substances identified at their peak in previous years have continued to be abused.
Synthetic cannabinoids are substances synthesized in laboratories that mimic the biological effects of THC, the main psychoactive ingredient in marijuana. Synthetic cannabinoids were introduced on the designer drug market in several European countries as "herbal incense" before the initial encounter in the United States by U.S. Customs and Border Protection (CBP) in November 2008. From 2009 to the present, misuse of synthetic cannabinoids has increased in the United States with law enforcement encounters describing synthetic cannabinoids applied onto plant material and in other designer drug products intended for human consumption.
Spraying or mixing the synthetic cannabinoids with plant material provides a vehicle for the most common route of administration - smoking (using a pipe, a water pipe, or rolling the drug-laced plant material in cigarette papers).
NM2201, 5F-AB-PINACA, 4-CN-CUMYL-BUTINACA, MMB-CHMICA and 5F-CUMYL-P7AICA have no accepted medical use in the United States. Use of 5F-CUMYL-P7AICA has not been documented in the United States yet, but its use has been reported to result in serious adverse events, including death, in other countries.

Synthetic Cannabinoids
It is easy to understand why these synthetic substitutes are alluring. They are easy to purchase, relatively inexpensive, produce a more potent high and don't emit the typical marijuana scent. And, they are much harder to detect in the urine or blood than marijuana. When you open a packet of a synthetic cannabinoid like K2 or Spice and pour the dried vegetation into your hand, it looks like marijuana. These dried leaves and stems can be inert or come from psychoactive plants like Wild Dagga. Some of these plants are contaminated with heavy metals, pesticides, mold or salmonella. However, synthetic cannabinoids are anything but natural. They are mass-produced overseas and then shipped in bulk to the U.S., where they are dissolved and then mixed with dried vegetation, which absorbs the liquid. This process is very imprecise, so the dose in one packet can differ greatly within or between batches. Synthetic cannabinoids are 30 times more likely to harm you than regular marijuana. Even with these risks, 7 percent of high school seniors and approximately 17 percent of adults have tried synthetic cannabinoids.

https://theconversation.com/why-synthetic-marijuana-is-so-risky-102224

(2014) Sold openly in stores, popular with kids and unpredictably dangerous, synthetic pot is just around the corner
The most complicated drug problem in the world right now isn't meth or cocaine or the heroin that's been making a comeback and killed actor Philip Seymour Hoffman. It is synthetic drugs, also known as legal highs or designer drugs. Five years ago, these substances were virtually unheard of. Now, say drug monitors and law-enforcement officials, they are spreading to eager buyers everywhere at an unprecedented speed. "It is widespread in scope. It is in every state," says Joseph Rannazzisi, head of the U.S. Drug Enforcement Administration group responsible for synthetics. "I don't recall any other drug issue where we had the same problem."
Mixed by chemists in labs, mostly in Asia, synthetics are chemical compounds designed to mimic the effects of naturally occurring drugs like marijuana and cocaine while staying just inside the law. Because the newest compounds don't yet appear on state and federal lists of illegal drugs, the sellers can market them as legal. As soon as authorities add a compound to the prohibited list, the chemists tweak the formula - ever so slightly - to make a new substance that purports to be legal.
Cannabinoids are now the most popular kind of synthetic, and the increasing legalization of pot may further burnish the myth that these chemicals are mostly harmless. But their effects, which are only beginning to be understood, can be unpredictable and dangerous. Emergency rooms and poison-control centers have reported synthetic-related kidney failure, seizures and psychoses.
https://time.com/magazine/us/57155/april-21st-2014-vol-183-no-15-u-s/
What are the effects of using synthetic cannabinoids like JWH-018, JWH-073, JWH-250 and others?
JWH-018, JWH-073, JWH-250 and other similar chemicals are the primary synthetic cannabinoid receptor agonists responsible for the euphoric and psychoactive effects that imitate marijuana and are among the numerous compounds found in herbal incense and smoke blends. These synthetic cannabinoids do not contain cannabis but produce effects reported up to 4 times the strength of THC/marijuana. Users indicate the high comes on slow at first, then with surprising potency. There have been many reports about the adverse effects including agitation, rapid heart rate, confusion, dizziness and nausea. In fact, the American Association of Poison Control Centers issued a warning about the dangers of synthetic marijuana products in March 2010. Long-term effects from these research chemicals are unknown.
Ok so for those of us that like tobacco you will enjoy an easy and stealth way to smoke JWH-073, JWH-018, JWH-200, and JWH-250 anywhere. The process for this one is very simple. Just take 3 mg or 4 mg of your favorite JWH and place it in the tip of your packed cigarette. The thing to do on this is keep the cigarette upright for the first hit. Since the JWH is in the tip it will fall out if you try and smoke it normally. Once you have ripped the JWH off the top you can finish the rest of your cigarette. By the end of the smoke you are baked. This is a great way to get high and walk through toon town at disneyland! Safe travels.
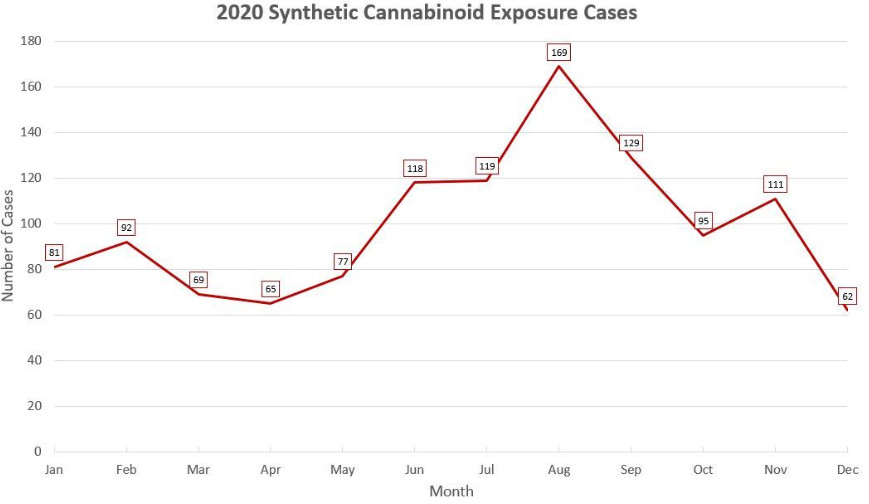
As of December 31, 2020, poison control centers have managed 1,187 calls for synthetic cannabinoid-related exposure cases.
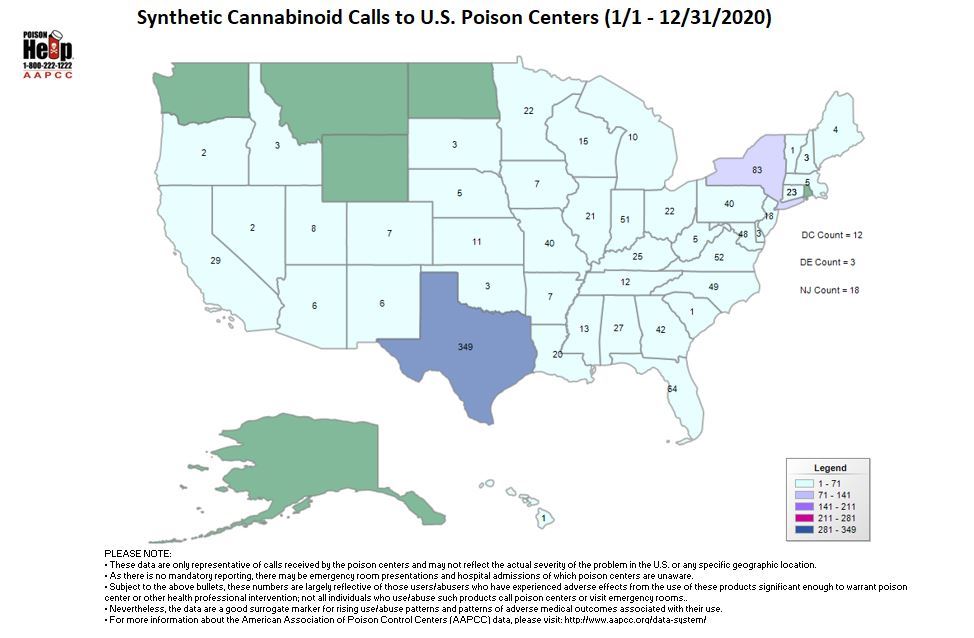
You can reach your local poison control center by calling the Poison Help hotline: 1-800-222-1222. To save the number in your mobile phone, text POISON to 797979.

https://aapcc.org/track/synthetic-cannabinoids
Synthetic Weed:
K2, Spice, and other synthetic cannabinoids are typically referred to as synthetic weed or marijuana, but this term is scientifically inaccurate. This lack of understanding has sparked confusion among those who might hear the term "synthetic cannabinoids" and instantly think about the perilous fake weed that has caused intense paranoia, hallucinations, and in some cases, overdoses. The full truth about synthetic cannabinoids, however, is buried somewhere underneath the nationwide synthetic weed scandal.The Difference Between Synthetic Weed and Synthetic Cannabinoids:
From a scientific perspective, synthetic weed or synthetic marijuana technically doesn't exist. In reality, synthetic cannabinoids are sprayed to dry herbs or plant material and marketed as an alternative to natural marijuana. Although both cannabis and synthetic weed bind to the cannabinoid receptors, cannabis and synthetic weed are not chemically related. The chemicals found in K2 or Spice have also proven to be much more potent, producing stronger and more intense effects.One study, published in Front Public Health on June 7, 2018, observed and compared the effects of synthetic with non-synthetic cannabinoid drugs, and found the synthetically produced substances appeared to produce more adverse effects in subjects. The researchers claimed that the "THC-like" effects synthetic cannabinoids were "more severe and enduring" than those from natural cannabinoids. Compared with cannabis and other psychoactive substances, synthetic cannabinoids were also associated with a greater risk of developing serious mental health disorder.
There are three different categories of synthetic cannabinoids:
 What is Synthetic Weed and Why is it Hazardous?
What is Synthetic Weed and Why is it Hazardous?
https://weedmaps.com/news/2019/06/what-is-synthetic-weed-cannabinoids-spice-danger/
Synthetic marijuana:
Is a common, but misleading, term that refers to a class of substances more accurately called cannabinoid receptor agonists or synthetic cannabinoids. Whereas marijuana usually refers to the dried flowered buds of the actual plant, which derives its main psychoactive effect through THC, synthetic cannabinoids get their name from their action on various cannabinoid receptors in the brain. People in public office, the media, and law enforcement often sacrifice accuracy for simplicity and use the term "synthetic marijuana" or the brand names of products sold, such as "Spice" or "K2," that are known to contain various synthetic cannabinoids.

https://drugpolicy.org/what-synthetic-marijuana


https://drugpolicy.org/sites/default/files/Synthetic_Cannabinoid_Fact_Sheet.pdf


https://psnc.org.uk/greater-manchester-lpc/wp-content/uploads/sites/118/2017/07/Spice-info-sheet1.3-Salford-1.pdf


https://www.drugfoundation.org.nz/assets/uploads/2013-uploads/20131129candhs06Bright.pdf


https://www.unodc.org/documents/scientific/Synthetic_Cannabinoids.pdf


https://www.emcdda.europa.eu/system/files/publications/2753/POD_Synthetic%20cannabinoids_0.pdf

https://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids_en

https://www.reddit.com/r/noids//

https://drugs-forum.com/tags/jwh/
Drugs-Forum Synthetic Cannabinoids:
https://drugs-forum.com/tags/synthetic-cannabinoids/
Top 10 Facts You Need to Know About Synthetic Cannabinoids: Not So Nice Spice:

 Top 10 Facts You Need to Know About Synthetic Cannabinoids: Not So Nice Spice (PDF 6 pages):
Top 10 Facts You Need to Know About Synthetic Cannabinoids: Not So Nice Spice (PDF 6 pages):
https://www.amjmed.com/article/S0002-9343(15)01008-6/pdf
False Advertising:
Synthetic cannabinoid products are often labeled "not for human consumption." Labels also often claim that they contain natural material taken from a variety of plants. However, the only parts of these products that are natural are the dried plant materials. Chemical tests show that the active, mind-altering ingredients are cannabinoid compounds made in laboratories.
Points to Remember:
- Synthetic cannabinoids refer to a growing number of human-made mind-altering chemicals sprayed on dried, shredded plant material or vaporized to produce a high.
- Synthetic cannabinoids are sometimes misleadingly called synthetic marijuana (or fake weed) because they act on the same brain cell receptors as THC, the mind-altering ingredient in marijuana.
- The effects of synthetic cannabinoids can be unpredictable and severe or even life-threatening.
- The only parts of synthetic cannabinoid products that are natural are the dried plant materials. Chemical tests show that their active ingredients are human-made cannabinoid compounds.
- Synthetic cannabinoid users report some effects similar to those produced by marijuana:
- Elevated mood
- Relaxation
- Altered perception
- Symptoms of psychosis
- Synthetic cannabinoids can also cause serious mental and physical health problems including:
- Rapid heart rate
- Vomiting
- Violent behavior
- Suicidal thoughts
- Synthetic cannabinoids can be addictive.
- Behavioral therapies and medications have not specifically been tested for treatment of addiction to these products.
- Overdoses can occur and can cause:
- Toxic reactions
- Raised blood pressure
- Reduced blood supply to the heart
- Kidney damage
- Seizures
- Deaths can occur when dangerous synthetic opioids, such as fentanyl, are added without the user knowing.
 Synthetic Cannabinoids (K2/Spice) DrugFacts:
Synthetic Cannabinoids (K2/Spice) DrugFacts:
https://www.drugabuse.gov/publications/drugfacts/synthetic-cannabinoids-k2spice

 Synthetic Cannabinoids (K2/Spice) Drug Facts (PDF 4 pages):
Synthetic Cannabinoids (K2/Spice) Drug Facts (PDF 4 pages):
https://www.drugabuse.gov/sites/default/files/drugfacts-synthcannabinoids.pdf
Synthetic cannabinoids are a class of molecules that bind to the same receptors to which cannabinoids (THC and CBD) in cannabis plants attach
Synthetic cannabinoids are typically not identified by the standard marijuana drug tests including the immunoassay test (EMIT), GC-MS screening, and multi-target screening by LC-GC/MS because those tests only detect the presence of THC and its metabolites. Although most synthetic cannabinoids are analogs of THC, they are structurally different enough.
- Designer drugs - designed in an attempt to avoid legal restrictions on cannabis
- Have been marketed as herbal incense, or herbal smoking blends
- Sold under common names like K2, Spice, and Synthetic Marijuana
- Often labeled "not for human consumption" for liability defense
- Usually smoked
Synthetic marijuana compounds began to be manufactured and sold in the early 2000s. From 2008 to 2014, 142 synthetic cannabinoids were reported
Reported user negative effects include palpitations, paranoia, intense anxiety, nausea, vomiting, confusion, poor coordination, and seizures. There have also been reports of a strong compulsion to re-dose, withdrawal symptoms, and persistent cravings.
There have been several deaths linked to synthetic cannabinoids.
| Some of the Synthetic Cannabinoids: |
|
|
5F-AB-PINACA
|
5F-AB-PINACA is an indazole-based synthetic cannabinoid developed by Pfizer in 2009 as an analgesic medication. In January 2015, AB-PINACA became a controlled substance in the USA and 5F-ABPINACA will be prohibited based on the analog law. 5F-AB-PINACA has been sold online as a designer drug.
 5-FLUORO-AB-PINACA:
5-FLUORO-AB-PINACA:
https://drugs.ncats.io/drug/3L83B2298V
- A synthetic cannabinoid
- Has been sold online as a designer drug
- Derived from a series of compounds originally developed by Pfizer in 2009 as an analgesic medication
5F-AMB
|
Synthetic cannabinoid which has been used as an active ingredient in synthetic cannabis products. It was first identified in Japan in early 2014.
5F-APINACA
|
World Health Organization 2016:
5F-APINACA is a psychoactive substance and has effects similar to THC.May produce:
- Nausea
- Vomiting
- Agitation
- Hallucinations
- Panic attacks
- Tachycardia
- Hypertension
- Occasionally chest pain
- Acute psychosis
- Seizures.
Clinical signs of toxicity (chest pain, tachycardia, hypertension, and agitation) following smoking of 5F-APINACA usually resolve within 4 to 10 hours and may require treatment with benzodiazepines.
Long term use of 5F-APINACA is characterized by:
Which are all exacerbated when attempting to reduce use.
- Loss of appetite
- Cognitive impairment
- Breathlessness
- Cardiac conditions requiring medication
- Skin ablations
- Tooth decay
- Lethargy
- Apathy
- Tremors
- Insomnia
Studies on abuse and dependence potential of 5F-APINACA have not been performed, but users reported a rapidly developing dependence (tolerance, compulsive re-dosing,craving). A variety of withdrawal symptoms have been reported, such as:
No therapeutic or medical use has been described for 5F-APINACA and 5F-APINACA is neither marketed as medicinal product, nor used for industrial purposes.
- Chest pains and pressure
- Tachycardia and palpitations
- Ongoing insomnia (for over 3 weeks)
- Anxiety
- Agitation
- Paranoia
For recreational use, herbs containing synthetic cannabinoids such as 5F-APINACA are smoked, often mixed with tobacco in joints, bongs and pipes.

 Excerpt (PDF 28 pages): https://www.who.int/medicines/access/controlled-substances/4.10_5F-APINACA_CritReview.pdf?ua=1
Excerpt (PDF 28 pages): https://www.who.int/medicines/access/controlled-substances/4.10_5F-APINACA_CritReview.pdf?ua=1
- An indazole-based synthetic cannabinoid
- Has been sold online as a designer drug
- First identified in South Korea
5F-CUMYL-P7AICA
|
- Synthetic cannabinoid
- Has been sold as a designer drug
- First identified by the EMCDDA in February 2015
5F-MDMB-PINACA
|
EMCDDA - Europol Joint Report 2017:
In its pure form 5F-MDMB-PINACA has been described as a white solid. It is poorly soluble in water.5F-MDMB-PINACA is typically found in herbal/plant material (including as commercially-branded 'legal high' products) and as a powder. To a lesser extent, other forms, such as liquids and blotters,have also been reported.
The most common way of using synthetic cannabinoids such as 5F-MDMB-PINACA is by smoking either ready-to-use or homemade 'smoking mixtures' as a cigarette ('joint') or by using a vaporizer,'bong', or pipe. Some synthetic cannabinoids, including 5F-MDMB-PINACA, have also been offered in the form of e-liquids for vaping in e-cigarettes. Additionally, users might also prepare 5F-MDMB-PINACA containing e-liquids at home. To a lesser extent, other routes of administration for synthetic cannabinoids have been reported; these include oral and rectal.
Limited information is available regarding the dose and the dose regimens of 5F-MDMB-PINACA.User reports specifically about 5F-MDMB-PINACA were not particularly revealing. It is not possible to discern the 'typical' dosages administered as most individuals use herbal smoking mixtures.Nonetheless, based on data from the analysis of some of these products, a gram of herbal material could contain more than 100 mg of 5F-MDMB-PINACA (and potentially other synthetic cannabinoids).These compounds may be active at less than 1 mg.
5F-MDMB-PINACA is more potent than MDMB-CHMICA
Onset of 1 to 5 minutes after smoking and effect duration of 1 to 2 hours. In some cases effects have been described to last over 10 to 15 hours.
World Health Organization 2017:
5F-ADB is a synthetic cannabinoid receptor agonist (SCRA) with an aminoalkylindazole structure used as an active ingredient of products sold as cannabis substitutes. 5F-ADB has no known therapeutic or medical use. In different regions it is being used and abused for non-medical purposes. Furthermore, some countries have put 5F-ADB under national control.When smoked, 5F-ADB produces cannabimimetic effects like delta9-tetrahydrocannabinol (THC). Doses needed to produce these effects are lower than for THC.
Many of the risks linked to cannabis use are also present in the case of 5F-ADB, among them complications in patients suffering from cardiovascular diseases and triggering of acute psychosis.

 World Health Organization 5F-ADB Review Report (PDF 16 pages):
World Health Organization 5F-ADB Review Report (PDF 16 pages):
https://www.who.int/medicines/access/controlled-substances/CriticalReview_5F-ADB.pdf
- An indazole-based synthetic cannabinoid
- Has been used as an active ingredient in synthetic cannabis products
- Has been sold online as a designer drug
- 5F-ADB is believed to be extremely potent based on the very low levels detected in tissue samples, and appears to be significantly more toxic than earlier synthetic cannabinoid drugs that had previously been sold.
5F-ADB was first identified in November 2014 from post-mortem samples taken from an individual who had died after using a product containing this substance. Subsequent testing identified 5F-ADB to have been present in a total of ten people who had died from unexplained drug overdoses in Japan between September 2014 and December 2014.
- In 2018, 5F-ADB was the most common synthetic cannabinoid to be identified in Drug Enforcement Administration seizures.
5F-PB-22
|
World Health Organization 2017:
5F-PB-22 (quinolin-8-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate) is a synthetic cannabinoid receptor agonist (SCRA) that had no history in the scientific literature until its detection emerged in 2013.This substance has been encountered as a synthetic constituent found in herbal smoking mixtures that are sold under a variety of brand names. It is common for retailers to purchase bulk quantities of the synthetic substance and add the synthetic material to plant matter that is then distributed onto the market. However, 5F-PB-22 is also available in powdered form as a "research chemical".
Various UN Member States reported the identification of 5F-PB-22 first in 2013 and data obtained from law enforcement suggest that 5F-PB-22 emerged and peaked in 2013 and 2014in the United States of America, which then dropped in the following years.

 Excerpt (PDF 31 pages): https://www.who.int/medicines/access/controlled-substances/CriticalReview_5FPB22.pdf?ua=1
Excerpt (PDF 31 pages): https://www.who.int/medicines/access/controlled-substances/CriticalReview_5FPB22.pdf?ua=1
- A designer drug
- Acts as a cannabinoid agonist
- Several deaths have been associated with its use
It appears to have been designed with an understanding of structure - activity relationships within the indole class of cannabinoids.
AB-CHMINACA
|
World Health Organization 2017:
AB-CHMINACA has no known therapeutic or medical use. In different regions it is being used and abused for non-medical purposes. Furthermore,some countries have put AB-CHMINACA under national control. When smoked, AB-CHMINACA produces cannabimimetic effects like THC.Doses needed to produce these effects are much lower than for THC. Many of the risks linked to cannabis use are also present in the case of AB-CHMINACA, among them complications in patients suffering from cardiovascular diseases and triggering of acute psychosis.
The abuse potential seems to be higher than for other synthetic cannibinoids.

 Excerpt (PDF 21 pages): https://www.who.int/medicines/access/controlled-substances/CriticalReview_ABCHMINACA.pdf?ua=1
Excerpt (PDF 21 pages): https://www.who.int/medicines/access/controlled-substances/CriticalReview_ABCHMINACA.pdf?ua=1
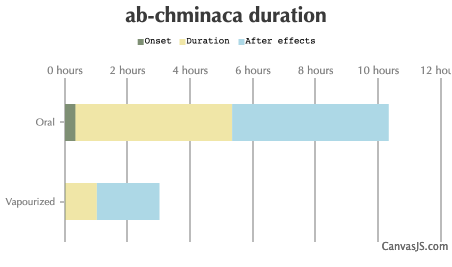
| Duration: A synthetic cannabinoid related to AB-FUBINACA which has had some mild popularity. There have been reports of death in overdose, similar to AB-FUBINACA and it has a low threshold dose. Exercise caution. | |||
| Route | Onset | Duration | After Effects |
|---|---|---|---|
| Tripsit Factsheets | |||

AB-CHMINACA Basic Information: http://drugs.tripsit.me/ab-chminaca | |||
| Oral: | 20-80 minutes | 5-12 hours | 5-12 hours |
| Vaporized: | 1-5 minutes | 1-2 hours | 2-4 hours |
| |||
EMCDDA - Europol Joint Report 2017:
In humans, AB-CHMINACA appears to cause effects that resemble those of cannabis and other synthetic cannabinoids. It shares some pharmacological similarities with THC, which is responsible for the major psychoactive effects of cannabis.AB-CHMINACA has been available in the European Union since at least February 2014. It has been detected in 24 Member States, Turkey and Norway. More than 4 000 seizures have been made within the European Union, which includes 43 kg of powder and 40 kg of herbal material which has been laced with AB-CHMINACA.
Thirty-one deaths with confirmed exposure to AB-CHMINACA have been reported by six Member States. In at least seven of the deaths, AB-CHMINACA was the cause of death or contributed to the death.

 European Monitoring Centre for Drugs and Drug Addiction AB-Chminaca (PDF 67 pages):
European Monitoring Centre for Drugs and Drug Addiction AB-Chminaca (PDF 67 pages):
http://www.emcdda.europa.eu/system/files/publications/9105/Risk%20assessment%20AB-CHMINACA.pdf
- An indazole-based synthetic cannabinoid
- Fully substitutes for delta9-THC in rat discrimination studies, while being 16x more potent
There have been a number of reported cases of seizures, deaths, and psychotic episodes in relation to this synthetic cannabinoid
AB-FUBINACA
|
AB-FUBINACA is a synthetic cannabinoid and it is a very potent agonist for the cannabinoid (CB1) receptor. AB-FUBINACA is designated as a Schedule I controlled substance in the United States.
 AB‐FUBINACA:
AB‐FUBINACA:
https://drugs.ncats.io/drug/I7PZF0KTFK
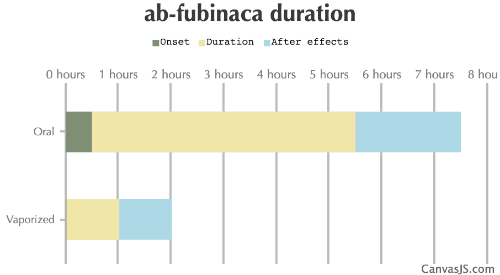
| Duration: Arguably the most common synthetic cannabinoid, AB-FUBINACA was originally developed by Pfizer as an analgesic, but has since abandoned for medical use. It has since found a following in the RC community, however it's extremely high potency and inclusion in synthetic blends makes it dangerous, and it has killed in overdose. Exercise caution. | |||
| Route | Onset | Duration | After Effects |
|---|---|---|---|
| Tripsit Factsheets | |||

Excerpt from http://drugs.tripsit.me/ab-fubinaca
| |||
| Oral: | 30-240 minutes | 5-15 hours | 2-10 hours |
| Vaporized: | 1-5 minutes | 1-2 hours | 1-2 hours |
| |||
Aliases:
| |||
| Effects: Euphoric, stimulating high, increases creativity and appetite, also short term memory deficits, similar to Cannabis | |||


https://www.deadiversion.usdoj.gov/drug_chem_info/spice/ab_fubinaca.pdf
Its use has been linked to hospitalizations and deaths
Mass overdoses:
On August 15th, 2018, 70 people within the city of New Haven, Connecticut started overdosing near Yale University campus. By the end of the week, the total number of overdosed had risen to over 100 people needing transport to local emergency rooms. Three men were arrested, charged as drug dealers selling synthetic cannabis which contained AB-FUBINACA. Almost all of the overdoses occurred on the New Haven Green, a large downtown park that is heavily traveled and very popular with the homeless population. There have been no deaths associated with these overdoses; however, several victims are in critical or life-threatening condition.Originally developed by Pfizer in 2009 as an analgesic medication but was never pursued for human use. In 2012, it was discovered as an ingredient in synthetic cannabinoid blends in Japan, along with a related compound AB-PINACA, which had not previously been reported.
AB-PINACA
|
World Health Organization 2017:
It is common for retailers to purchase bulk quantities of the synthetic substance and add the synthetic material to plantmatter that is then distributed onto the market. However, AB-PINACA is also available in powdered form as a "research chemical". The identification of AB-PINACA was reported first in 2012 by Japan and more forensic detections began to emerge in other countries in 2013. Data from law enforcement suggest that AB-PINACA was one of the most prevalent substances in the United States of America in 2014, which dropped again in the following years.AB-PINACA was shown to be 2-to 14-fold more potent than THC
AB-PINACA, in its pure form but mostly as a synthetic constituent added to a plant matrix (e.g. damiana (Turnera diffusa) or marshmallow (Althaea officinalis), is normally smoked but reliable data about dosage are normally unavailable.

 Excerpt (PDF 31 pages): https://www.who.int/medicines/access/controlled-substances/CriticalReview_ABPINACA.pdf
Excerpt (PDF 31 pages): https://www.who.int/medicines/access/controlled-substances/CriticalReview_ABPINACA.pdf
- First identified as a component of synthetic cannabis products in Japan in 2012
- Originally developed by Pfizer in 2009 as an analgesic medication
- Fully substitutes for delta-9-THC in rat discrimination studies, while being 1.5x more potent
There have been a number of reported cases of deaths and hospitalizations in relation to this synthetic cannabinoid.
ADB-FUBINACA
|
ADB-FUBINACA is a synthetic cannabinoid, a Schedule I drug. ADB-FUBINACA acts as a high potency agonist of cannabinoid CB1 receptor. The compound considered to be a component of illicit smoking mixtures "spice".
 N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide:
N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide:
https://drugs.ncats.io/drug/05235E1S2O
World Health Organization 2018:
- ADB-FUBINACAis a synthetic cannabinoid that likely shares a profile of centrally mediated effects with other synthetic cannabinoids, including THC-like intoxication.
- The most likely route of administration for ADB-FUBINACA is inhalation via smoking the chemical after it has been sprayed on plant material or vaping it after formulation in liquid.
- Little information is available about the pharmacokinetics of ADB-FUBINACA.
- Although sparse in number, extant case studies suggest that acute administration of ADB-FUBINACA has contributed to severe adverse reactions in humans up to, and including, death.
- ADB-FUBINACA was first identified in samples originating from Japan in 2013.

 World Health Orgination (PDF 18 pages):
World Health Orgination (PDF 18 pages):
https://www.who.int/medicines/access/controlled-substances/ADB_Fubinaca.pdf

https://erowid.org/experiences/subs/exp_ABFUBINACA.shtml
In 2018, it was the third-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration
- A designer drug
- Identified in synthetic cannabis blends in Japan in 2013
At least an additional 8 deaths in Hungary in 2015 are linked to the usage of this material, all deaths were youngsters below 21
ADB-PINACA
|
A cannabinoid designer drug that is an ingredient in some synthetic cannabis products. ADB-PINACA has been linked to multiple hospitalizations and deaths due to its use.
AM-2201
|
Many users report that the intensity of AM-2201 in high doses can be overwhelming. This may in part be due to the extreme potency, thereby making overdose a significant danger. AM-2201 seems to be very unforgiving in high doses compared to other cannabinoids.
Effects of AM-2201
Positive:
- Euphoria
- Body high
- Relaxation
- Laughter
- Enhancement of colors
- Dry mouth
- Increase in appetite
- Lethargy
- Especially high potency creates a high risk of overdose
- Paranoia
- Psychosis and delusional thoughts (overdose)
- Nausea
- Dizziness

https://drugs-forum.com/wiki/AM-2201


https://www.who.int/medicines/areas/quality_safety/4_7_EPR_1.pdf

https://erowid.org/experiences/subs/exp_AM2201.shtml

https://www.soft-tox.org/files/designer_drugs/AM-2201.pdf
- A recreational designer drug
- Comparable to the potency of JWH-018
Convulsions have been reported including at doses as low as 10 mg
AM-694
|
AM-694 is considered to be a strong psychoactive substance, thus users whom have a known mental illness should avoid this compound as it may further exaggerate the symptoms of such illness. Furthermore AM-694 is active in the sub milligram dosage range, with the potential for an overdose to occur being considerably high especially if the compound is prepared with inaccurate or miscalibrated equipment, because of this a highly accurate and calibrated scale capable of weighing in single milligram denominations is required in order to safely handle this compound.
Effects of AM-694:
Positive:
- Euphoria
- Sedation
- Feelings of joy
- Laughter
- Relaxation
- Body high
- Dry mouth
- Hunger
- Internal auditory hallucinations
- Open and closed eye visual hallucinations
- Anxiety
- Body Load
- Paranoia
- Terrifying hallucinations
- Derealization
- Depersonalization
- Dissociation
- Schizophrenic like through process

https://drugs-forum.com/wiki/AM-694

https://www.soft-tox.org/files/designer_drugs/AM-694.pdf
- A designer drug
- It is used in scientific research for mapping the distribution of cannabinoid receptors in the body
APINACA
|
A second generation of synthetic cannabinoids
World Health Organization 2014:
There is little information available for APINACA. It belongs to the category of synthetic cannabinoid receptor agonists (SCRAs). APINACA is a psychoactive substance and has effects similar to THC. It has been detected in herbal products marketed under a variety of names via the Internet and in specialised shops. The quantity of APINACA among the different packages may vary considerably.Detailed information on the toxic effects of APINACA is not available. In general SCRAs may produce:
Intoxications have led to hospital admissions, but the psychoactive SCRA is rarely identified. Studies on abuse and dependence potential of APINACA have not been performed, but considering its close pharmacological resemblance to THC, abuse of APINACA is likely to occur.
- Nausea
- Vomiting
- Agitation
- Hallucinations
- Panic attacks
- Tachycardia
- Hypertension
- Occasionally chest pain
- Myoclonia
- Acute psychosis
- Seizures

 World Health Organization Apinaca Review Report (PDF 18 pages):
World Health Organization Apinaca Review Report (PDF 18 pages):
https://legal-high-inhaltsstoffe.de/sites/default/files/uploads/akb-48_-_apinaca.pdf
Named after popular Japanese Girl band AKB48 (https://en.wikipedia.org/wiki/AKB48)
First identified by laboratories in Japan in March 2012 as an ingredient in synthetic cannabis smoking blends
CBL2201
|
Similar properties to the closely related 5F-PB-22 and NNE1. NM-2201 was linked to an incident in December 2015 where 25-30 people in Ocala, FL were taken to hospitals after experiencing seizures
CP-47-497
|
- Initially developed by Pfizer in the 1980's.
CP-47-497 can be administered via vaporization/smoking, where the compound is either vaporized in its pure form with the byproduct of combustion being inhaled, or the compound is laced over some sort of plant material and then smoked out of a pipe or similar smoking utensils. Alternatively CP-47-497 can be administered via oral consumption with the assistance of a carrier which is high in fat content. Examples of such carriers would be whole milk, butter, peanut butter, olive oil, or cream.
There are several notable dangerous associated with CP-47-497 which one should be aware of. CP-47-497 is considered to be a research chemical, thus the long term and short term effects/side effects have yet to be determined. CP-47-497 is considered to be a psychoactive drug thus users with a known mental illness should avoid this compound as it may aggravate the symptoms of such illnesses. Inhaling the byproduct of combustion, no matter what the source may be, can and will cause harm to one's mouth, sinus cavities, esophagus, and lungs, the amount of harm caused as a result of inhaling combusted CP-47-497 has not yet been determined.

https://drugs-forum.com/wiki/CP_47_497
CP 47,497:
- A cannabinoid receptor agonist drug
- Developed by Pfizer in the 1980s
- It has analgesic effects
- Used in scientific research
CP-47-497 C8 HOMOLOGUE
|

https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3094488/
Cannabicyclohexanol:
- A cannabinoid receptor agonist drug
- Developed by Pfizer in 1979
- Was the main active ingredient in the herbal incense product Spice
- Several times more potent than the parent compound CP-47-497
JWH 018
|
JWH compounds are named after the scientist who first synthesized them: John W. Huffman
JWH-018 is one of the original synthetic cannabinoids.
World Health Organization 2014:
JWH-018 is an aminoalkylindole used as an active ingredient of products sold as cannabis substitutes. When smoked, JWH-018 produces cannabimimetic effects in doses lower than the doses of THC needed to produce effects of similar strength (higher potency). Many of the risks linked to cannabis use are also present in the case of JWH-018, among them complications in patients suffering from cardiovascular diseases and triggering of acute psychosis.Abuse potential and dependence potential seem to be similar to cannabis. One of the major differences between cannabis and this synthetic cannabinoid is the greater acute toxicity of JWH-018. Due to its full agonistic action at the CB1 receptor, the side effects of higher doses can be life-threatening. This is aggravated by the fact that dosing is very difficult due to changing contents of active ingredients in different products, different batches of the same product and even within one packet. Regarding chronic toxicity, risks are very difficult to estimate on the basis of the available data. However, there are concerns about potential carcinogenic effects.
Reports suggest a duration of action for JWH-018 of approximately 1-2 hours when smoked
It gained popularity in late 2008 when German chemists found it as a chemical within the popular synthetic cannabis blend Spice, which had been sold in numerous countries around the world since 2002. Cannabinoids are commonly smoked or vaporized to achieve a quick onset of effects and rapid offset. JWH-018 is orally active when dissolved in a lipid, which can increase the duration significantly. Like other cannabinoids, it is insoluble in water but dissolves in ethanol and lipids.
Unlike cannabis, the chronic abuse of synthetic cannabinoids has been associated with multiple deaths and more dangerous side effects and toxicity in general. Therefore, it is very strongly discouraged to take this substance for extended periods of time or in excessive doses.
Tolerance to many of the effects of JWH-018 develops with prolonged and repeated use. This results in users having to administer increasingly large doses to achieve the same effects. After that, it takes about 3 - 7 days for the tolerance to be reduced to half and 1 - 2 weeks to be back at baseline (in the absence of further consumption). JWH-018 presents cross-tolerance with all cannabinoids, meaning that after the consumption of JWH-018 all cannabinoids will have a reduced effect.

https://psychonautwiki.org/wiki/JWH-018
Synthetic Marijuana: Six Things You Should Know About Smoking JWH-018:
- Not all incense blends are created equal. The herbal blend doesn't matter compared to the amount of JWH-018 in the blend.
- JWH-018 is more expensive than real pot.
- Herbal incense blends are harsh. smoking herbal incense can make your throat burn and your lungs ache, hours after the last hit.
- JWH-018 does not mix well with alcohol. Most drugs don't mix well with alcohol, but having a glass or two of wine with JWH-018 can exacerbate hangovers and cause headaches at the base of the skull that last for hours.
- It doesn't give you "the munchies."
- The high last no more than 30 minutes. The effects of smoking JWH-018 don't last long - probably an average of 10 minutes, or half an hour at best for the more potent herbal blends.
JWH-018 is a full agonist synthetic cannabinoid with a high binding affinity to CB1 and CB2 cannabinoid receptors. JWH-018 has not been used in therapy. Many of the risks linked to cannabis use are also present in the case of JWH-018, among them complications in patients suffering from cardiovascular diseases and triggering of acute psychosis. JWH-018 has not been used in therapy. Studies in mice showed anti-inflammatory and cancer chemopreventive properties of JWH-018.

https://drugs-forum.com/threads/jwh-018-experiences.88133/

https://www.420magazine.com/community/forums/jwh-018.625/

https://www.bluelight.org/xf/threads/mega-jwh-018-thread.407913/

https://erowid.org/experiences/subs/exp_JWH018.shtml
JWH-018:
- An analgesic chemical from the naphthoylindole family
- It produces effects similar to those of tetrahydrocannabinol (THC), leading to its use in synthetic cannabis products that in some countries are sold legally as "incense blends".
- The analgesic effects of cannabinoid ligands, mediated by CB1 receptors are well established in treatment of neuropathic pain, as well as cancer pain and arthritis.
These compounds work by mimicking the body's naturally-produced endocannabinoid hormones, which are biologically active and can exacerbate or inhibit nerve signaling.
On December 15, 2008, it was reported by German pharmaceutical companies that JWH-018 was found as one of the active components in at least three versions of the grey market drug Spice, which has been sold as an incense in a number of countries around the world since 2002. An analysis of samples acquired four weeks after the German prohibition of JWH-018 took place found that the manufacturers had shortened the alkyl chain by one carbon to circumvent the ban.
JWH 019
|
Drugs-Forum JWH-019:
- It kinda creeps on and doesn't have the initial 018 rush. It doesn't really have that uneasy,paranoia of an OD kinda feeling with higher doses.You can feel it pleasantly underneath the eye's and brain.Nice body stone from the chest pushing inwards, and also the shins warping back..From the shins inwards kinda feel.
- I have experience with JWH-019 (he may be the one you referenced) and would advise against it because it is somewhat underwhelming. There are better cannabinoids for the money.
- Totally spot on commentary! A independent tester has confirmed that JWH-019 was a complete dud and provided no buzz or high whatsoever. Of course various types of Cannabinoids will affect each individual differently. But my suggestion would be to get your favorite vendor to offer a small free or low cost sample to try before buying this particular JWH in larger quantities.
- I have experience with JWH-019 (he may be the one you referenced) and would advise against it because it is somewhat underwhelming. There are better cannabinoids for the money.

https://drugs-forum.com/threads/jwh-019-experiences.95662/

https://erowid.org/experiences/subs/exp_JWH019.shtml
JWH-019:
JWH 073
|
JWH-073, a synthetic cannabinoid, is an analgesic chemical from the naphthoylindole family that acts as a neutral antagonist of CB1 cannabioid receptor and binder of CB2 cannabinoid receptor. JWH-073 is being used as a recreational drug in Spice products, and is a controlled substance in USA.
JWH-073 is one of the main compounds found in synthetic marijuana all around the world, including the name brands "Spice" and "K2". Developed by John W. Huffman, JWH-073 is a synthetic analogue similar, yet stronger, to the effects that found in THC, the active component in cannabis (weed). Because of its potency, JWH-073 has been identified by the DEA as extremely dangerous and carries the high potential for abuse. Many countries, and many states in the USA, have banned the substance. After the use of the chemical JWH-018 was banned in many countries, synthetic marijuana manufacturers began replacing the substance with JWH-073. The effects of the two chemicals are almost identical, but their structural differences make the use of JWH-073 possible.
http://detoxanswers.com/questions/833/what-is-jwh-073
One of the original synthetic cannabinoids.
JWH-073 has no known therapeutic or medical use
World Health Organization 2016:
When smoked, JWH-073 produces cannabimimetic effects like THC. Doses needed to produce these effects are in the same range as THC doses. Many of the risks linked to cannabis use are also present in the case of JWH-073, among them complications in patients suffering from cardiovascular diseases and triggering of acute psychosis. The abuse potential potential seems to be similar to cannabis. Compared to JWH-018, JWH-073 is less potent and tends to show lower efficacy. JWH-018 is an analogue of JWH-073.

 Excerpt (PDF 29 pages):
Excerpt (PDF 29 pages):
https://www.who.int/medicines/access/controlled-substances/4.11_JWH-073_CritReview.pdf?ua=1
The abbreviation JWH stands for John W. Huffman, one of the inventors of the compound.
JWH-073 gained popularity in April 2009, when it was claimed by chemists at the University of Freiburg to have been found in a "fertilizer" product called "Forest Humus", along with another synthetic cannabinoid, CP 47,497. It was subsequently found as a chemical within the popular synthetic cannabis blend Spice, which had been sold in numerous countries around the world since 2002.

https://psychonautwiki.org/wiki/JWH-073

https://erowid.org/experiences/subs/exp_JWH073.shtml

https://www.shroomery.org/forums/showflat.php/Number/11399605
JWH-073:
- An analgesic chemical from the naphthoylindole family
- JWH-073 has been shown to produce behavioral effects very similar to THC in animals. A search in the literature yielded no published studies of the effects of JWH-073 in humans, but these studies in animals suggest with high probability that JWH-073 produces effects very similar to those of THC in humans.
On 20 April 2009, JWH-073 was claimed by researchers at the University of Freiburg to have been found in a "fertiliser" product called "Forest Humus", along with another synthetic cannabinoid (C8)-CP 47,497. These claims were confirmed in July 2009 when tests of Spice product, seized after the legal ban on JWH-018 had gone into effect in Germany, were shown to contain the unregulated compound JWH-073 instead.
JWH 081
|
- As mentioned in my experience JWH-081 is a slightly beigh/off-white crystalline powder with a consistency similar to organic or unbleached sugar. It appears not to be particularly hygroscopic and seems to be reasonably stable when kept sealed in a dry, dark place at room temperature. Vaporizing 2-3 milligrams using a lighter and a heat-safe glass pipe produces effects with a slow onset (2-3 minutes to fully develop) lasting for approximately 3-4 hours although I usually performs 2-3 additional booster hits over the course of an hour (spread out usually by 5 or more minutes) with 1-2 additional hits approximately every hour (as desired). Effects include a heavy physical stoning, enhanced sense of relaxation and a mild (although note-worthy) euphoria/pleasure. Side-effects include slight munchies, decreased motor control, glossy eyes and somewhat extreme dry-mouth. The dry mouth is worth mentioning on its own because it seems to parallel the pleasure caused by the substance and often I will find himself enjoying the dryness of his mouth with a glass of water well within arms reach and not take a drink. It's funny behavior I hasn't experienced previously or with any other substance.

https://drugs-forum.com/threads/jwh-081-experiences.82832/
Description: JWH 081 is an analytical reference standard categorized as a synthetic cannabinoid, It has been found in Spice/K2-type herbal blends and may have neurotoxic properties. JWH 081 is regulated as a Schedule I compound in the United States. This product is intended for research and forensic applications. JWH-081 is discontinued (DEA controlled substance).

https://www.medkoo.com/products/20356

https://erowid.org/experiences/subs/exp_JWH081.shtml
JWH-081:
- An analgesic chemical from the naphthoylindole family
- JWH-081 may be neurotoxic to animals when administered in high doses.
JWH 122
|
Independent lab tests of the holiday bread sold in early January by a Santa Ana, CA, bakery and linked to reports of dizziness, palpitations and other symptoms show that it was probably deliberately contaminated with synthetic cannabinoid, an artificial version of the main chemical ingredient in marijuana. More than 40 people in Southern California were reportedly sickened after eating the special holiday bread called Rosca de Reyes. Local health officials said several people complained of various symptoms, including dizziness, blurred vision, anxiety, palpitations and numbness, after eating the bread from Cholula's Bakery in Santa Ana, CA. The chemical found, JWH-122, is used to make synthetic pot, which is also called "spice," "incense" and "K2." It is commonly diluted with water, sprayed onto herbs and smoked like marijuana. The side effects of using synthetic marijuana can be more severe than those from using real marijuana and include hallucinations, aggressive behavior and hypertension. Smoking the synthetic marijuana known as "spice" has sent thousands of people, mainly teenagers, to hospital emergency rooms across the country, according to the Drug Enforcement Administration.

https://www.foodsafetynews.com/2015/02/tests-show-synthetic-drug-contaminated-holiday-bread-from-ca-bakery/

https://drugs-forum.com/tags/jwh-122/

https://erowid.org/experiences/subs/exp_JWH122.shtml
JWH-122:
- It is a methylated analogue of JWH-018
In January 2015, over 40 people were reportedly sickened after eating a holiday bread called Rosca de reyes purchased at a bakery in Santa Ana, CA that was laced with JWH-122.
JWH 200
|
Initially discovered by Dr. John W. Huffman, JWH-200 is a CB1/CB2 cannabinoid receptor agonist which possess a variety of psychoactive effects in humans at doses as low as 1 milligram. JWH-200 selectively binds to the CB1 receptor with IC50 values of 42 nM, almost equal to that of tetrahydrocannabinol (THC), additionally JWH-200 possesses analgesic effects which are 3x greater than that of THC.

https://drugs-forum.com/wiki/JWH-200

https://erowid.org/experiences/subs/exp_JWH200.shtml
JWH-200:
- An analgesic chemical from the aminoalkylindole family
JWH 203
|
- Here's some more info about JWH-203. I have it on good authority that it does get you pretty high but causes a lot of drowsiness and the worst thing is the effect on hearing. I'm referring to oral use. The source of my information doesn't smoke it because he finds it pretty much unsmokable. What happens, at least with a significant dosage, is that your ears start ringing, meaning you hear a constant high pitch sound for the first while and then your hearing drastically decreases in volume and things start sounding tinny and higher. For instance, Anderson Cooper on CNN has a fairly high voice normally but on JWH-203 he will sound like Mickey Mouse, and Wolf Blitzer will also have a high voice even though it's normally husky. Obviously, driving on this would be a really bad idea.

https://drugs-forum.com/threads/jwh-203-experiences.149948/


https://air.unimi.it/retrieve/handle/2434/159566/254182/JWH%20203.pdf
JWH-203:
- An analgesic chemical from the phenylacetylindole family
- Has been sold as an ingredient of synthetic cannabis smoking blends
JWH 250
|
JWH-250 or (1-pentyl-3-(2-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors, with a Ki of 11 nM at CB1 and 33 nM at CB2. JWH-250 does not have any therapeutic application, however it is found in many herbal products that are smoked for their psychoactive effects. JWH-250 is a controlled substance by FDA.
JWH 250 has potency comparable to that of THC.
- My pet turtle Orion is a regular MJ smoker and recently acquired 100mg of this substance for testing purposes. About 2 minutes after inhaling, Orion felt a warm glowing feeling in his spine and back of his head, spreading quickly to his entire head. Colors are slightly enhanced, and it's almost like the contrast has been turned up a bit. There doesn't seem to be any couch-locking effect, as Orion felt like he had lots of energy for the duration of the high. He initially felt very little or absent body high, as the initial part of the experience seems to be solely a head high. About 15 minutes in, Orion felt a slight trippy feel to the experience, almost like the onset of a mushroom trip. After 30 minutes or so, a body high starts creeping in and sticks around for the next hour and a half or so. Orion was pleased to note no CNS depression at higher doses (ie: above 5mg) with this compound, unlike JWH-018 which seems to produce slight CNS depression at doses above 5mg. After about 1.5 hours, the effects seem to completely subside back to baseline.

https://drugs-forum.com/threads/jwh-250-experiences.104271/
World Health Organization 2014:
JWH-250 is a psychoactive substance and has effects similar to those of THC. It has been detected in herbal products marketed under a variety of names via the Internet and in specialised shops. The quantity of JWH-250 among the different packages may vary considerably.

 World Health Organization (PDF 20 pages):
World Health Organization (PDF 20 pages):
https://legal-high-inhaltsstoffe.de/sites/default/files/uploads/jwh-250.pdf
JWH-250 is a synthetic cannabinoid receptor agonist that has been detected in products since the late 2000s. It was created by Dr. John W. Huffman and a report on it was released in 2005 by Huffman et al.
A few years after it was first mentioned in the literature, JWH-250 appeared on the market, with the first detection coming from German police in May 2009. They found the drug in an herbal smoking mixture.
It's also been detected in powders and blends sold via the internet to researchers in Japan. Those samples contained 77-165 mg per product.
Because of the way it's been sold, it's likely people have encountered JWH-250 without realizing it.

https://thedrugclassroom.com/video/jwh-250/

https://erowid.org/experiences/subs/exp_JWH250.shtml
JWH-250:
- An analgesic chemical from the phenylacetylindole family
Samples of JWH-250 were first identified in May 2009 by the German Federal Criminal Police, as an ingredient in new generation "herbal smoking blends" that had been released since the banning of the original ingredients (C8)-CP 47,497 and JWH-018.
An ELISA immunoassay technique for detecting JWH-250 in urine has been reported.
Unlike many of the older JWH series compounds, this compound does not have a naphthalene ring, instead occupying this position with a 2'-methoxy-phenylacetyl group, making JWH-250 a representative member of a new class of cannabinoid ligands. Other 2'-substituted analogues such as the methyl, chloro and bromo compounds are also active and somewhat more potent.
JWH 398
|
JWH-398:
- An analgesic chemical from the naphthoylindole family
This synthetic chemical compound was identified by the EMCDDA as an ingredient in three separate "herbal incense" products purchased from online shops between February to June 2009.
MAB-CHMINACA
|
EMCDDA - Europol Joint Report 2017:
ADB-CHMINACA appears to cause effects that resemble those of cannabis and other synthetic cannabinoids.ADB-CHMINACA has been available in the European Union since at least August 2014 and has been detected in 18 Member States, Turkey and Norway. More than 600 seizures have been made within the European Union, which includes 7 kg of powder and 11 kg of herbal material which has been laced with ADB-CHMINACA.
This herbal material is typically sold as smoking mixtures; the products are marketed as 'legal' replacements to cannabis. Due to the way that these products are produced, it appears that users are at risk of serious poisoning.
There are indications that the AB-CHMINACA available on the market was synthesised by chemical companies based in China.Twelve deaths with confirmed exposure to ADB-CHMINACA have been reported by three Member States. In at least nine of the deaths, ADB-CHMINACA was the cause of death or contributed to the death.

 Excerpt (PDF 15 pages):
Excerpt (PDF 15 pages):
http://www.emcdda.europa.eu/system/files/publications/5485/2017.4966_TDAS17006ENN_PDFWEB.pdf
World Health Organization 2018:
First documented in international patent WO 2009/106980-A2 issued to Ingrid Buchler and colleagues at Pfizer on September 3, 2009. ADB-CHMINACA is also referred to as MAB-CHMINACA.Case studies and reports of mass intoxication indicate that acute administration of ADB-CHMINACA has the potential to produce severe adverse reactions in humans up to, and including, death. Symptoms of ADB-CHMINACA overdose may include tachycardia, unresponsiveness, seizures, delirium, slurred speech, vomiting, agitation and combativeness. In the spring of 2015, ADB-CHMINACA was associated with several drug-induced clusters of severe illness and death in the U.S. It has also been associated with 13 deaths in the European Union and one death in Japan.
White powder or crystalline solid.
The acute psychological effects of synthetic cannabinoids (including ADB-CHMINACA)may resemble those reported during acute intoxication with cannabis, ranging from a relaxed and unfocused euphoria to feelings of distress (e.g., confusion, anxiety, and fear). Time perception may be distorted, and in susceptible individuals, hallucinations, paranoia, and more serious psychiatric disorder may occur. Physical effects may include bloodshot eyes (as is characteristic of THC), tachycardia, nausea, vomiting, seizures, and impaired motor performance. Because synthetic cannabinoids are usually more potent (and also may be more efficacious) than phytocannabinoids, their effects occur at lower doses, and overdose may be more common, as suggested by increased reports of deaths and serious adverse reactions with this class of cannabinoids as compared to cannabis.11, 17-20Since users usually are unaware of which synthetic cannabinoid is contained in a product, they may administer a chemical with greater potency than the chemical contained in previous products. Further, the chemical may not be evenly distributed throughout the plant material, creating "hot spots" containing higher concentrations of synthetic cannabinoid. For these reasons, dose (in THC equivalents) often exceeds intended dose. Contaminants (e.g., pesticides, heavy metals, rodent feces) may also be present and may contribute to adverse reactions.Case studies and reports of mass intoxication suggest that acute administration of ADB-CHMINACA has the potential to produce severe adverse reactions in humans up to, and including, death.
ADB-CHMINACA:
- An indazole-based synthetic cannabinoid
- Originally developed by Pfizer in 2009 as an analgesic medication
- It was identified in cannabinoid blends in Japan in early 2015
There have been a number of reported cases of deaths and hospitalizations in relation to this synthetic cannabinoid.
MDMB-CHMICA
|
World Health Organization 2016:
Structurally, MDMB-CHMICA shares some similarities with JWH-018 and AM-2201.MDMB-CHMICA has no known approved medical or industrial use. MDMB-CHMICA has been detected in seized materials in several countries including Europe and USA. In Europe,it was first detected in 2014.
Higher potency compared to delta-9-tetrahydrocannabinol (THC) and possibly to JWH-018.
Because of lack of clear dosages in the contents of the products sold containing MDMB-CHMICA,users are at risk of experiencing life threatening side effects associated with MDMB-CHMICA. Deaths have been associated with MDMB-CHMICA.
MDMB-CHMICA is an odourless white crystalline solid (in pure form). It has been seized in powder, tablet and herbal mixtures form. MDMB-CHMICA has been detected in seized herbal mixtures of green/brown color.
EMCDDA - Risk Assessment 2017:
Some users report that the effects of smoking herbal mixtures containing MDMB-CHMICA are more pronounced in comparison to cannabis. A review of 36 user experiences on user websites found that the most commonly self-reported effects were euphoria, visual hallucinations, anxiety, paranoia, amnesia, sense of doom, and auditory hallucinations (in decreasing order). Other effects that have been reported include: feeling of warmth, laughing, changes in mood, disorientation, confusion, lack of concentration, vertigo, agitation, fear/panic, psychosis, dissociation, violent behaviour, aggression and 'acute behavioural disturbances'. These are similar to psychological and behavioural effects reported for other synthetic cannabinoid receptor agonists. Many of the effects mentioned relate to neuropsychiatric symptoms.MDMB-CHMICA has been available on the drug market in the European Union since at least August 2014
Clinical features were generally consistent with cannabis-like toxicity but included life threatening conditions.
MDMB-CHMICA has no recognized human or veterinary medical use.

 MDMB-CHMICA Risk Assessments (PDF 51 pages):
MDMB-CHMICA Risk Assessments (PDF 51 pages):
http://www.emcdda.europa.eu/system/files/publications/4093/TDAK16002ENN_PDFWEB.pdf

 MDMB-CHMICA Joint Reports (PDF 20 pages):
MDMB-CHMICA Joint Reports (PDF 20 pages):
https://www.emcdda.europa.eu/publications/joint-reports/mdmb-chmica_en
2015 Article:
A new legal high which can apparently kill in "two puffs" has been found in the UK. It's the first time authorities have detected the drug - nicknamed 'Black Mamba' - here, but it is believed to have already killed six people in Europe. The drug - also known as synthetic cannabis MDMB-CHMICA - is designed to be smoked, but experts warn it can have fatal consequences. This drug in particular said to cause irregular heartbeat, nausea, paranoia, confusing, vomiting, breathlessness and hallucinations, and has already been linked to four deaths in Sweden, and two in Germany. Grim. Health experts say the discovery represents a "significant and growing problem" - and unsurprisingly, there are now urgent calls for the drug to be banned.

https://www.cosmopolitan.com/uk/reports/news/a36967/mdmb-legal-high/

https://erowid.org/experiences/subs/exp_MDMBCHMICA.shtml
71 serious adverse events, including 42 acute intoxications and 29 deaths (Germany (5), Hungary (3), Poland (1), Sweden (9), United Kingdom (10), Norway (1)) which occurred in 9 European countries between 2014 and 2016 have been associated with MDMB-CHMICA. Over 3600 MDMB-CHMICA seizures between 2014 and 2016 in 19 member states of the European Union have been reported to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA).
MDMB-FUBINACA
|
There have been a large number of reported cases of deaths and hospitalizations in relation to this synthetic cannabinoid, mainly in Russia and Belarus. MDMB-FUBINACA was first reported in 2014 and quickly gained a reputation as the most deadly synthetic cannabinoid drug sold by 2015.
MMB-CHMICA
|
MMB-CHMINACA
|

https://www.reddit.com/r/noids/comments/7t1qvs/i_tried_mmbchminaca/

https://erowid.org/experiences/subs/exp_MDMBCHMICA.shtml

https://www.reddit.com/search/?q=MMB-CHMINACA

https://drugs-forum.com/threads/mmb-chminaca-information-expectations-experiences.253270/

https://www.drugsdata.org/view.php?id=3988
NABILONE
|
Nabilone is a synthetic cannabinoid approved under the brand name cesamet for treatment of severe nausea and vomiting associated with cancer chemotherapy. Nabilone is an orally active which, like other cannabinoids, has complex effects on the central nervous system (CNS). It has been suggested that the antiemetic effect of nabilone is caused by interaction with the cannabinoid receptor system, i.e. the CB (1) receptor, which has been discovered in neural tissues.
 Nabilone:
Nabilone:
https://drugs.ncats.io/drug/2N4O9L084N
This medication is used to relieve severe nausea and vomiting caused by cancer chemotherapy. It is used when other drugs to control nausea and vomiting have not been successful. Nabilone is a man-made drug similar to the natural substances found in marijuana. It is believed to work by decreasing the signals in the brain that lead to nausea and vomiting.

https://www.medicinenet.com/nabilone-oral/article.htm
Nabilone is used to treat the nausea and vomiting that may occur during treatment with cancer medicines. It is only used when other kinds of medicine for nausea and vomiting do not work.Nabilone is only available with your doctor's prescription.
This product is available in the following dosage forms: Capsule
Nabilone will add to the effects of alcohol and other central nervous system (CNS) depressants (medicines that make you feel drowsy or less alert). Some examples of CNS depressants are antihistamines or medicine for hay fever, other allergies, or colds; sedatives, tranquilizers, or sleeping medicine; prescription pain medicines, including other narcotics; barbiturates; medicine for seizures; muscle relaxants; or anesthetics, including some dental anesthetics. Check with your doctor before taking any of the above while you are taking this medicine.
If you think you or someone else may have taken an overdose, get emergency help at once. Taking an overdose of this medicine or taking alcohol or CNS depressants with this medicine may cause severe mental effects. Symptoms of overdose include changes in mood; confusion; difficulty in breathing; hallucinations (seeing, hearing, or feeling things that are not there); nervousness or anxiety (severe); and fast or pounding heartbeat.
Pediatric:
Studies with this medicine have only been done in adult patients, and there is no specific information comparing use of nabilone in children with use in other age groups.Caution should be used in prescribing nabilone to children under the age of 18 years due to its mind and mood-altering effects.
Geriatric:
Fast or pounding heartbeat, feeling faint or lightheaded, and unusual tiredness or weakness may be especially likely to occur in elderly patients, who are usually more sensitive than younger adults to the effects of nabilone.Also, the effects this medicine may have on the mind may be of special concern in the elderly.
Therefore, older people should be watched closely while taking this medicine.Other Medical Problems:
Make sure you tell your doctor if you have any other medical problems, especially:
- Alcohol abuse (or history of)
- Drug abuse or dependence (or history of) - Dependence on nabilone may develop.
- Emotional problems
- Heart disease
- Low blood pressure
- Manic or depressive states
- Mental illness (severe)
- Schizophrenia - Nabilone may make the condition worse.
- Kidney problems
- Liver problems - Nabilone has not been studied in patients with these conditions
 Nabilone (Oral Route) (Cesamet):
Nabilone (Oral Route) (Cesamet):
https://www.mayoclinic.org/drugs-supplements/nabilone-oral-route/description/drg-20064938
What side effects can Nabilone cause?
Nabilone may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away: headache, dizziness, unsteady walking, drowsiness, sleep problems, weakness, dry mouth, changes in appetite, nausea, high or elevated mood, difficulty concentrating, anxiety, confusion,
depression
Some side effects can be serious. If you experience any of these symptoms, call your doctor immediately:
- fast heartbeat
- hallucinations (seeing things or hearing voices that do not exist)
- difficulty thinking clearly and understanding reality
| Maximum Dosage: | ||
| Group | Dose | Notes |
|---|---|---|
| Prescribers Digital Reference | ||

Nabilone - Drug Summary: https://www.pdr.net/drug-summary/Cesamet-nabilone-692 | ||
| Adults: | 6 mg/day PO | |
| Elderly: | 6 mg/day PO | |
| Adolescents: | 3 mg/day PO | has been studied |
| Children: > 30 kg: | 3 mg/day PO | has been studied |
| Children: 18-30 kg: | 2 mg/day PO | has been studied |
| Children: < 18 kg: | 1 mg/day PO | has been studied |
| Side Effects: |
| Get emergency medical help if you have any of these signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat. |
|---|
| RxList |
Stop using nabilone and call your doctor at once if you have:
|
Common side effects may include:
|
| This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
Liver:
Nabilone is associated with a minimal rate of serum enzyme elevations during therapy and has not been linked to cases of clinically apparent liver injury with jaundice.
Nabilone Hepatotoxicity:
Serum aminotransferase elevations during nabilone therapy are not common, generally mild and similar in to the rate in controls who are receiving cancer chemotherapy. There have been no convincing cases of clinically apparent liver injury attributable to nabilone published in the literature and, thus, significant liver injury from nabilone must be exceeding rare, if it occurs at all.
 Nabilone Overview:
Nabilone Overview:
https://www.ncbi.nlm.nih.gov/books/NBK547865/

https://reference.medscape.com/drug/cesamet-nabilone-342048

https://www.drugs.com/comments/nabilone/

https://www.reddit.com/search/?q=Nabilone
Prescribed for:
What Conditions does it Treat?
- Nausea and vomiting caused by cancer drugs
Uses:
This medication is used to treat severe nausea and vomiting caused by cancer drug treatment (chemotherapy).Nabilone is a man-made drug similar to the natural substances found in marijuana (cannabis).
It is works by decreasing the signals in the brain that lead to nausea and vomiting.Before using:
Tell your doctor or pharmacist your medical history, especially of:
- Liver disease
- High blood pressure
- Heart disease
- Mental/mood conditions (such as mania, depression, schizophrenia)
- Personal or family history of a substance use disorder (such as overuse of or addiction to drugs/alcohol)
Precautions:
- This drug may make you dizzy or drowsy
- Alcohol or marijuana (cannabis) can make you more dizzy or drowsy
- Do not drive, use machinery, or do anything that needs alertness until you can do it safely.
- Avoid alcoholic beverages.
- Talk to your doctor if you are using marijuana (cannabis).
This product also contains alcohol. Caution is advised if you have diabetes, liver disease, or any other condition that requires you to limit/avoid alcohol in your diet. Ask your doctor or pharmacist about using this product safely.
Read Reviews (7):
https://www.webmd.com/drugs/drugreview-144706-nabilone-oral.aspx?drugid=144706&drugname=nabilone-oral
 Nabilone Capsule (Cesamet):
Nabilone Capsule (Cesamet):
https://www.webmd.com/drugs/2/drug-144706/nabilone-oral/details

https://erowid.org/experiences/subs/exp_Nabilone.shtml


https://www.cadth.ca/media/pdf/htis/oct-2011/RC0306-000%20Nabilone%20for%20chronic%20pain.pdf


https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018677s011lbl.pdf
Nabilone, sold under the brand name Cesamet among others, is a synthetic cannabinoid with therapeutic use as an antiemetic and as an adjunct analgesic for neuropathic pain. It mimics tetrahydrocannabinol (THC), the primary psychoactive compound found naturally occurring in Cannabis. Nabilone is used to treat nausea and vomiting in people under chemotherapy. Nabilone has shown modest effectiveness in relieving fibromyalgia. Nabilone is sometimes used for nightmares in post-traumatic stress disorder, but there have not been studies longer than nine weeks, so effects of longer-term use are not known. Nabilone has also been used for medication overuse headache.Nabilone was originally developed by Eli Lilly and Company; Lilly received FDA approval in 1985 to market it, but withdrew that approval in 1989 for commercial reasons. Valeant Pharmaceuticals acquired the rights from Lilly in 2004. Valeant tried and failed to get the medication approved in 2005 and then succeeded in 2006.
NM2201
|
Synthetic cannabinoid that presumably has similar properties to the closely related 5F-PB-22 and NNE1.
NM-2201 was linked to an incident in December 2015 where 25-30 people in Ocala, FL were taken to hospitals after experiencing seizures.
PARAHEXYL
|
Definition of Synhexyl:
A compound derived from dibenzo-pyran that is said to have a euphoriant action more powerful than that of cannabis and is used experimentally in the treatment of depressive mental states.

https://www.merriam-webster.com/dictionary/synhexyl
1948 paper on the Psychological Effects of Synhexyl:
Summary:
The effects of synhexyl, a homologue of one of the active principles of marihuana, have been investigated in a group of patients with predominantly depressive characteristics. The symptoms produced were those of general impairment of cerebral function and mild clouding of consciousness. No evidence that the drug is valuable as a treatment for depression was obtained. The symptoms produced were in contrast to those of benzedrine in the same group of subjects. These findings are discussed in relation to the mechanism of action of the drug.


https://jnnp.bmj.com/content/jnnp/11/4/271.full.pdf
A synthetic homologue of THC, which was invented in 1949 during attempts to elucidate the structure of THC, one of the active components of cannabis. Parahexyl is similar in both structure and activity to THC. Parahexyl produces effects typical of other cannabinoid receptor agonists in animals. It has a somewhat higher oral bioavailability than THC itself but is otherwise very similar. Parahexyl was occasionally used as an anxiolytic in the mid-20th century, the dosage ranging from 5 mg to 90 mg.
PB-22
|
PB-22, a recreational "designer drugs", is a cannabimimetic agent, it is a full agonist of cannabinoid receptors. PB-22 has an EC50 of 5.1 nM for human CB1 receptors, and 37 nM for human CB2 receptors. PB-22 produces bradycardia and hypothermia in rats at doses of 0.3 - 3 mg/kg, suggesting potent cannabinoid-like activity. PB-22 was designated as a Schedule I controlled substance in the United States.
PB-22 is an analog of JWH 018 which differs by having 8-hydroxyquinoline replacing the naphthalene group of JWH 018.

https://www.adooq.com/pb-22.html
WARNING This product is not for human or veterinary use.
A designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013. PB-22 represents a structurally unique synthetic cannabinoid chemotype rather than the precedented ketone of JWH-018 and its analogs, or the amide of APICA and its analogs.History:
PB-22 was originally developed by New Zealand legal highs company Stargate International in 2012 as SGT-21, intended to be a structural hybrid of QMPSB and JWH-018. However, no intellectual property protection was applied for and the compound quickly became subject to widespread grey-market sales outside the control of the inventors.
SGT-78
|
EMCDDA - Europol Joint Report (2017):
In March 2017, the EMCDDA and Europol examined the available information on the new psychoactive substance 1-(4-cyanobutyl)-N-(2-phenylpropan-2-yl)indazole-3- carboxamide, commonly known as CUMYL-4CN-BINACA, through a joint assessment. The two organisations launched a data collection on this substance for the preparation of a Joint Report as stipulated by Article 5.1 of the Council Decision. The Joint Report was submitted to the Council, the Commission and the European Medicines Agency on 3 July 2017.Conclusion:
CUMYL-4CN-BINACA is a synthetic cannabinoid and a CB1 receptor agonist. It shares some pharmacological similarities with delta9-tetrahydrocannabinol (THC), which is responsible for the major psychoactive effects of cannabis. In humans, CUMYL-4CN-BINACA appears to cause effects that resemble those of cannabis and other synthetic cannabinoids. CUMYL-4CN-BINACA has been available in the European Union since at least October 2015 and has been detected in nine Member States and Turkey. More than 270 seizures have been made within the European Union, which includes more than 50 kg of powder and 3.6 kg of herbal material which has been laced with CUMYL-4CN-BINACA. This herbal material is typically sold as smoking mixtures; the products are marketed as 'legal' replacements to cannabis. Due to the way that these products are produced, it appears that users are at risk of serious poisoning.

 CUMYL-4CN-BINACA Joint Reports (PDF 15 pages):
CUMYL-4CN-BINACA Joint Reports (PDF 15 pages):
https://www.emcdda.europa.eu/system/files/publications/5486/2017.4965_TDAS17005ENN_PDFWEB.pdf

 Risk Assessment Report (2018) (PDF 60 pages):
Risk Assessment Report (2018) (PDF 60 pages):
https://www.emcdda.europa.eu/system/files/publications/8621/Risk_assessment_CUMYL_BINACA.pdf
World Health Organization 2018:Illicit manufacture and traffic:
CUMYL-4CN-BINACA has been detected in 11 Member States of the European Union and in the U.S. Underreporting is likely due to lack of routine screening for the compound. Synthesis of the compound occurs predominantly in chemical companies in China, with subsequent processing and packaging within the country to which it is shipped. In 2016, CUMYL-4CN-BINACA was among the top five synthetic cannabinoids seized in powder form.

 Critical Review Report: CUMYL-4CN-BINACA (PDF 19 pages):
Critical Review Report: CUMYL-4CN-BINACA (PDF 19 pages):
https://www.who.int/medicines/access/controlled-substances/Cumyl_4cn_binaca.pdf
- Synthetic cannabinoid
- Has been sold online as a designer drug
CUMYL-4CN-BINACA is metabolized to produce cyanide, raising concerns about liver toxicity
SR-18
|
WARNING: This product is not for human or veterinary use
Has been found as an ingredient of "herbal" synthetic cannabis blends
- A synthetic cannabinoid
- An analogue of JWH-250 and can be expected to be less potent than JWH-250
SR-19
|
WARNING: This product is not for human or veterinary use

https://drugs-forum.com/threads/rcs-4-4-methoxyphenyl-1-pentyl-1h-indol-3-yl-methanone-drug-info.139435/
- A synthetic cannabinoid
- A potent cannabinoid receptor agonist
- RCS-4 was banned in Sweden on 1 October 2010 as a hazardous good harmful to health, after being identified as an ingredient in "herbal" synthetic cannabis products
- Outlawed in Denmark March 2011
- In August 2011 New Zealand
- As of October 2015 China
THJ-2201
|
Dangerous Interactions:

Stimulants increase anxiety levels and the risk of thought loops which can lead to negative experiences

https://psychonautwiki.org/wiki/THJ-2201
THJ-2201:
- An indazole-based synthetic cannabinoid
- Has been sold online as a designer drug
- It is a structural analog of AM-2201
THJ-2201 has been linked to at least one hospitalization and death due to its use.
UR-144
|
XLR-11 was named after the first USA-developed liquid fuel rocket for aircraft (https://en.wikipedia.org/wiki/Reaction_Motors_XLR11)
UR-144 is a potent synthetic cannabinoid designed by Abbott Laboratories as a CB2 selective agonist for pain management and other indications. UR-144 preferentially binds the peripheral CB2 receptor (Ki = 1.8 nM) over the central CB1 receptor (Ki = 150 nM). Pre-clinical studies of nociceptive and neuropathic pain have shown that CB2-selective ligands are analgesics without causing the adverse side effects linked with CB1 receptor activation. However, UR-144 has no therapeutic application. According to the FDA (US Food and Drug Administration) there are currently no approved or on-going drug applications for the medical use of UR-144.
Synthetic Cannabinoids (UR144/XLR11)
Synthetic Cannabinoids are chemical compounds that mimic the effects of THC, the main active ingredient of cannabis. They bind to the cannabinoid receptors in the brain and were developed to treat pain. The two most common synthetic cannabinoids were JWH-018 and JWH-073. New versions of these include AM1248, AKB48, UR144 and XLR11. UR144 and XLR11 are the new generation of synthetic cannabinoids and are chemically different to the first generation. New generations of synthetic cannabinoids are continuously emerging to replace the synthetic cannabinoids that have been made illegal. The napthlene ring in JWH-018 is substituted with a tetramethylcyclopropyl group to form UR144. XLR11 is the fluorinated version of UR144.

https://thedrugclassroom.com/video/ur-144/

https://erowid.org/experiences/subs/exp_UR144.shtml

https://erowid.org/experiences/subs/exp_XLR11.shtml
| Link to this page Synthetic Cannabinoids |