[ Home ] [ Controlled Substances ] [ Prescription ]
PERAMPANEL
|
A seizure is a short episode of symptoms which is caused by a burst of abnormal electrical activity in the brain. Generalised seizures occur if you have a burst of abnormal electrical activity which spreads throughout the brain. Generalised seizures affect consciousness and may cause a convulsion. With a focal seizure, the burst of electrical activity stays in one part of the brain and therefore you tend to have localised or 'focal' symptoms. Because different parts of the brain control different functions, your symptoms will depend on which part of your brain is affected. Focal seizures can sometimes develop into seizures which affect all of your brain. These are called secondary generalised seizures.
Perampanel is prescribed alongside other anti-epileptic medicines. It works by stabilising the electrical activity in your brain.

https://patient.info/medicine/perampanel-for-epilepsy-fycompa
Perampanel is the generic name of a seizure medicine with the brand name Fycompa. The name or look may be different in other countries. The dose usually will be the same.
Used to treat:
- Temporal Lobe Epilepsy
- Focal Impaired Awareness or Complex Partial Seizures
- Secondarily Generalized Seizures or Bilateral Tonic Clonic Seizure
- Focal Aware or Simple Partial Seizure
- Tonic-clonic Seizures
Fycompa is approved for use:
- Alone or with other seizure medicines in adults and children aged 12 years and older with focal onset (partial) seizures.
- With other seizure medicines (as add-on therapy) in adults and children aged 12 years and older with generalized onset tonic clonic seizures.
| Fycompa Tablets: | |
|---|---|
 | 2 mg - orange color, '2' on one side, '275' on the other |
 | 4 mg - red color, '4' on one side, '277' on the other |
 | 6 mg - pink color, '6' on one side, '294' on the other |
 | 8 mg - purple color, labeled '8' on one side and '295' on the other |
 | 10 mg - green color, labeled '10' on one side and '296' on the other |
 | 12 mg - blue color, labeled '12' on one side and '297 on the other |
Forms:
Perampanel also comes as an oral suspension, is dose of 0.5 milligram for each milliliter (0.5 mg/ml). Use the adaptor and syringe that comes with the suspension to measure each dose.

https://www.epilepsy.com/medications/perampanel
2015:
It was approved as adjunctive therapy for PGTC seizures in patients with epilepsy 12 years and older. To date, perampanel is approved in 55 countries and has been used to treat more than 200,000 patients worldwide across all indications.
September 2018:
Perampanel was approved for monotherapy and adjunctive use in pediatric patients 4 years and older for the treatment of POS with or without SGS. Perampanel was first approved in 2012 for adjunctive use in POS in patients 12 years and older.
2019:
Perampanel (Fycompa) was found to be effective in seizure control and response with a favorable tolerability outcome regardless of the first-line concomitant antiepileptic drug (AED) at baseline in patients older than 12 years with partial-onset seizures (POS), with or without secondary generalized seizures (SGS). "These data support the use of perampanel as first adjunctive therapy in patients with POS, with or without SGS, regardless of the first-line AED."

https://www.pharmacytimes.com/news/perampanel-found-to-be-effective-in-seizure-control-in-patients-with-partial-onset-seizures
Perampanel (trade name Fycompa) is an antiepileptic drug developed by Eisai Co. that acts as a selective non-competitive antagonist of AMPA receptors, the major subtype of ionotropic glutamate receptors. Although the mechanism of action through which perampanel exerts its antiepileptic effect has not been fully elucidated, this agent antagonizes the AMPA subtype of the excitatory glutamate receptor found on postsynaptic neurons in the central nervous system (CNS). This antagonistic action prevents AMPA receptor activation by glutamate and results in the inhibition of neuronal excitation, repetitive neuronal firing, and the stabilization of hyper-excited neural membranes. Glutamate, the primary excitatory neurotransmitter in the CNS, plays an important role in various neurological disorders caused by neuronal hyperexcitation. The drug is currently approved, for the control of partial-onset seizures, in those of both sexes who suffer from epilepsy and who are 12 years of age and older, by the Food and Drug Administration. Perampanel is also approved for the treatment of primary generalized tonic-clonic seizures in patients with epilepsy aged 12 years and older. It is designated as a Schedule III controlled substance by the Drug Enforcement Administration. Perampanel has been studied in other clinical indications including Parkinson's disease.
 Perampanel:
Perampanel:
https://drugs.ncats.io/drug/H821664NPK
Maximum Dosage:
| Maximum Dosage: | |
|---|---|
| Prescribers Digital Reference | |

Fycompa - Drug Summary: https://www.pdr.net/drug-summary/Fycompa-perampanel-3449 | |
| Adults: | 12 mg/day PO. |
| Geriatric: | 12 mg/day PO. |
| Adolescents: | 12 mg/day PO. |
| Children: | 4 to 12 years: 12 mg/day PO. |
| Children: | 1 to 3 years: Safety and efficacy have not been established. |
| Infants: | Safety and efficacy have not been established. |
| Neonates: | Safety and efficacy have not been established. |
Prescribed for:
- A type of seizure called a focal seizure
- Additional medication for tonic-clonic epilepsy
Used for:
Perampanel is used to treat certain types of seizures (including focal and generalized tonic-clonic seizures). Perampanel belongs to a class of drugs known as anticonvulsants.Before using:
Tell your doctor or pharmacist your medical history, especially of:
- Liver problems
- Kidney problems
- Mental/mood disorders
- Ppersonal or family history of a substance use disorder (such as overuse of or addiction to drugs/alcohol)
- Galactose intolerance (such as Lapp lactase deficiency or glucose-galactose malabsorption)
Precautions:
- This drug may make you dizzy or drowsy or blur your vision.
- Alcohol or marijuana (cannabis) can make you more dizzy or drowsy.
- Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely.
- Avoid alcoholic beverages.
- Talk to your doctor if you are using marijuana (cannabis).
 Perampanel Tablet (Fycompa):
Perampanel Tablet (Fycompa):
https://www.webmd.com/drugs/2/drug-165538/perampanel-oral/details
Important Information:
Some people taking perampanel have had serious psychotic effects, especially when starting perampanel or changing doses. Stay alert to changes in your mood or symptoms.
Call your doctor right away if you have any changes in mood or behavior changes, personality changes, thoughts about suicide, or thoughts about hurting others. Your family or other caregivers should also be alert to changes in your mood or symptoms.Avoid driving or hazardous activity until you know how perampanel will affect you. Dizziness or drowsiness can cause falls, accidents, or severe injuries.
Drinking alcohol with this medicine can cause side effects.
Interactions:
Drug Interactions (272) Alcohol/Food Interactions (3) Disease Interactions (5)
What other drugs will affect Perampanel?
Using perampanel with other drugs that make you drowsy can worsen this effect. Ask your doctor before using opioid medication, a sleeping pill, a muscle relaxer, or medicine for anxiety or seizures. Sometimes it is not safe to use certain medications at the same time. Some drugs can affect your blood levels of other drugs you take, which may increase side effects or make the medications less effective.Tell your doctor about all your other medicines, especially:
This list is not complete. Other drugs may affect perampanel, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here.
- birth control pills
- rifampin
- St. John's wort
- other seizure medicine:
- carbamazepine
- oxcarbazepine
- phenytoin
A total of 272 drugs are known to interact with Perampanel.
- 2 major drug interactions
- 256 moderate drug interactions
- 14 minor drug interactions
 Perampanel Information:
Perampanel Information:
https://www.drugs.com/mtm/perampanel.html
FYCOMPA Interactions:
- Antagonized by moderate and strong CYP3A4 inducers:
- Carbamazepine
- Phenytoin
- Oxcarbazepine
- Topiramate
- Rifampin
- St. John's wort
- Caution with concomitant CNS depressants:
- Benzodiazepines
- Narcotics
- Barbiturates
- Sedating antihistamines
- Avoid alcohol
- Reduces effectiveness of levonorgestrel-containing contraceptives; use additional non-hormonal form during and for 1 month after discontinuation.

https://www.empr.com/drug/fycompa/
Pediatric:
Appropriate studies have not been performed on the relationship of age to the effects of perampanel to treat partial onset seizures in children younger than 4 years of age or to treat generalized tonic-clonic seizures in children younger than 12 years of age.Safety and efficacy have not been established.
Geriatric:
Appropriate studies performed to date have not demonstrated geriatric-specific problems that would limit the usefulness of perampanel in the elderly. However, elderly patients are more likely to have unwanted effects, which may require an adjustment in the dose for patients receiving perampanel.Other Interactions:
- Ethanol
Other Medical Problems:
The presence of other medical problems may affect the use of this medicine. Make sure you tell your doctor if you have any other medical problems, especially:
- Behavior or mood changes (eg, aggression, anger)
- Coordination problems (eg, walking, balance)
- Depression
- Dizziness - Use with caution. May make these conditions worse
- Kidney disease
- Liver disease - Use with caution. The effects may be increased because of slower removal of the medicine from the body
- Kidney disease, severe
- Liver disease, severe
- Patients receiving hemodialysis - Use is not recommended in patients with these conditions
 Perampanel (Oral Route) (Fycompa):
Perampanel (Oral Route) (Fycompa):
https://www.mayoclinic.org/drugs-supplements/perampanel-oral-route/description/drg-20075877
| Side Effects: |
| Get emergency medical help if you have signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat. |
|---|
| RxList |
Fycompa (perampanel tablets, for oral use): https://www.rxlist.com/fycompa-drug.htm |
| Seek medical treatment if you have a serious drug reaction that can affect many parts of your body. Symptoms may include: skin rash, fever, swollen glands, muscle aches, severe weakness, unusual bruising, or yellowing of your skin or eyes. |
| Report any new or worsening symptoms to your doctor, such as: mood or behavior changes, anxiety, fear, panic attacks, trouble sleeping, or if you feel irritable, agitated, hostile, aggressive, restless, hyperactive (mentally or physically), or have thoughts about suicide or hurting yourself or someone else. |
Call your doctor at once if you have:
|
| Accidental falls may occur more often in elderly patients who take perampanel. Use caution to avoid falling or accidental injury while taking this medicine. |
Common side effects may include:
|
| This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
What is the most important information I should know about perampanel?
Some people taking perampanel have had serious psychotic effects, especially when starting this medicine or changing doses. Stay alert to changes in your mood or symptoms.Call your doctor right away if you have any:
Your family or other caregivers should also be alert to changes in your mood or symptoms.
- Changes in mood
- Behavior changes
- Personality changes
- Thoughts about suicide
- Thoughts about hurting others
| Black Box Warnings: |
|---|

Fycompa (perampanel) (Rx): https://reference.medscape.com/drug/fycompa-perampanel-999784 |
|
Serious or life-threatening psychiatric and behavioral adverse reactions including aggression, hostility, irritability, anger, and homicidal ideation, and threats reported May occur with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression Advise patients and caregivers to contact a healthcare provider immediately if any of these reactions or changes in mood, behavior, or personality that are not typical for the patient are observed Closely monitor patients particularly during the titration period and at higher doses Reduce dose if psychiatric and behavioral reactions occur and discontinue if symptoms are severe or worsen |
IMPORTANT WARNING:
People who have taken perampanel have developed serious or life-threatening changes in their mental health and behavior, especially increased hostility or aggression toward others. Tell your doctor if you have or have ever had any type of mental illness or aggressive behavior. However, you should know that it is possible to develop these changes in mental health and behavior while taking perampanel even if you have never had any problems with mental health or behavior in the past. You, your family, or your caregiver should call your doctor right away if you experience any changes in mood, behavior, or personality during your treatment with perampanel or for up to a month after stopping your treatment. Your doctor will monitor your mental health closely during your treatment with perampanel, especially when you begin taking this medication and any time that your dose is changed.If you experience any of the following symptoms, call your doctor immediately:
Your doctor may decrease your dose or stop your medication.
- Aggression
- Hostility
- Anger
- Anxiety
- Irritability
- Suspicious or distrustful behavior
- Confusion
- Memory problems
- Thinking about harming or killing others or threatening or trying to do so
Liver:
Perampanel has not been associated with serum aminotransferase elevations during therapy, and clinically apparent liver injury from perampanel has yet to be reported and must be rare, if it occurs at all.
Perampanel Hepatotoxicity:
Limited data are available on the hepatotoxicity of perampanel. In clinical trials, therapy with perampanel was not associated with an increased frequency of serum aminotransferase elevations as compared to placebo treatment, and there were no reported instances of clinically apparent liver injury. No individual case reports of perampanel hepatotoxicity have been published since its general clinical availability. Perampanel has been implicated in rare instances of severe cutaneous hypersensitivity reactions including DRESS syndrome, which can be associated with variable degrees of liver injury. Thus, clinically apparent liver injury due to perampanel may occur, but must be very rare.E Likelihood score: E (unlikely cause of clinically apparent liver injury).
 Perampanel Overview:
Perampanel Overview:
https://www.ncbi.nlm.nih.gov/books/NBK548508/
Breastfeeding:
Summary of Use During Lactation:
No information is available on use of perampanel during breastfeeding.If perampanel is required by the mother, it is not necessarily a reason to discontinue breastfeeding, but monitor the infant for drowsiness, agitation, adequate weight gain, and developmental milestones, especially in younger, exclusively breastfed infants and when using combinations of drugs.
Alternate Drugs to Consider:
- (Seizure Disorder) Carbamazepine
- Divalproex
- Gabapentin
- Lamotrigine
- Oxcarbazepine
- Phenytoin
- Valproic Acid
 Perampanel Drug Levels and Effects:
Perampanel Drug Levels and Effects:
https://www.ncbi.nlm.nih.gov/books/NBK500746/
Perampanel and Contraception:
Find out what types of contraception may work well for you if you take perampanel (12mg or more daily).

https://www.epilepsy.org.uk/info/contraception/perampanel-more-than-12mgs-daily
2012 FDA Clinical Pharmacology Review of Fycompa:
Executive Summary:
The sponsor is seeking approval of Fycompa (perampanel) as an adjunctive therapy for the treatment of partial-onset seizures with or without secondarily generalized seizures in patients aged 12 years and older.
The proposed formulations are film-coated oral tablets with strengths of 2, 4, 6, 8, 10 and 12 mg. The sponsor's proposed dosing regimen is: Fycompa should be taken once daily before bedtime; start with a dose of 2 mg/day; the dose may be increased based on clinical response and tolerability by an increment of 2 mg/day to a dose of 4 mg to 12 mg/day. The maximum recommended daily dose is 12 mg once daily. Dose increases should occur at weekly intervals and no more frequently than that.


https://www.fda.gov/media/84995/download


https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202834s011lbl.pdf
Abstract:
Objective:
The objective of this study was to evaluate the efficacy and tolerability of perampanel (PER) in late adjunctive treatment of focal epilepsy. We assessed outcomes
- According to patients' clinical profiles and the broad mechanism of action (MoA) of concomitant antiepileptic drugs (AEDs)
- The effects of PER on adverse events, irritability, mood, and quality of life (QOL).
Highlights:
- Patients with highly refractory epilepsy may experience complete seizure freedom after 12 months maintenance on perampanel
- Patients who have experienced six or more months of seizure freedom are more likely to achieve seizure freedom on perampanel
- Patients may experience improved mood, irritability and quality of life when maintained on perampanel for at least six months

https://www.epilepsybehavior.com/article/S1525-5050(19)30790-5/fulltext
Perampanel has a novel mechanism of action:
Despite best medical management with available anti-seizure medications, approximately 20-40 percent of patients with epilepsy remain refractory to available anti-epileptic drugs. Therefore, newer pharmacological agents with novel mechanisms have been sought to address the unmet need. Perampanel (Fycompa. Eisai, Inc.) is recently available for use in the United States and has been in use in Germany since September of 2012. Based on data from three randomized clinical trials, perampanel was approved for adjunctive treatment of patients who suffer from partial epilepsy with or without secondary generalization who are at least 12 years old.
An epilepsy specialist reviews the role of a new AMPA receptor agonist for epilepsy.

https://practicalneurology.com/articles/2014-july-aug/perampanel-novel-mechanism-for-the-management-of-epilepsy
December 2013 DEA schedules perampanel as a controlled substance (Schedule 3):
Scheduling Conclusion:
Based on consideration of all comments, the scientific and medical evaluation and accompanying recommendation of the HHS, and based on the DEA's consideration of its own eight-factor analysis, the DEA finds that these facts and all relevant data constitute substantial evidence of potential for abuse of perampanel. As such, the DEA is scheduling perampanel as a controlled substance under the CSA.

https://www.deadiversion.usdoj.gov/fed_regs/rules/2013/fr1202.htm
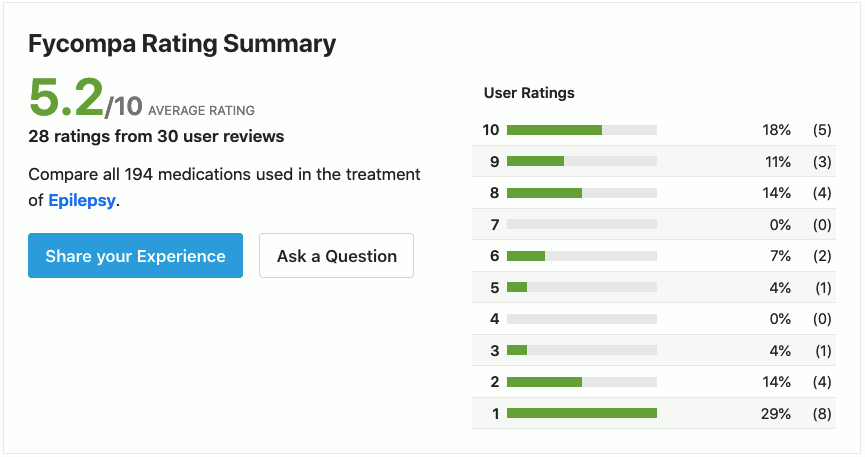

https://www.drugs.com/comments/perampanel/fycompa-for-epilepsy.html

https://www.reddit.com/search/?q=fycompa
Perampanel Reddit Search:
https://www.reddit.com/search/?q=perampanel

https://www.medicines.org.uk/emc/product/7874/smpc#gref

https://www.fycompa.com/hcp/mechanism-of-action
An antiepileptic drug. Used in addition to other drugs to treat partial seizures and generalized tonic-clonic seizures for people older than 12 years. It was first approved in 2012 and as of 2016 its optimal role in the treatment of epilepsy relative to other drugs was not clear. It was the first antiepileptic drug in the class of selective non-competitive antagonist of AMPA receptors. The drug label has a black box warning that the drug cause may cause serious psychiatric and behavioral changes; it may cause homicidal or suicidal thoughts. These events occurred in people who had no history of such issues, as well as people who had such a history. Other side effects have included dizziness, somnolence, vertigo, aggression, anger, loss of coordination, blurred vision, irritability, and slurred speech.Perampanel reduced the effectiveness of levonorgestrel oral contraceptives by about 40%. Women who may get pregnant should not take it as studies in animals show it may harm a fetus. It is not recommended for women of child-bearing age not taking contraception.
Perampanel is liable to be abused; very high doses produced euphoria responses similar to ketamine, although subjects liked it less and had experienced it more negatively than ketamine; it produced dissociative effects similar to ketamine.
Perampanel for epilepsy: Still no proof of added benefit - The drug perampanel (trade name Fycompa) has been approved since July 2012 as adjunctive therapy for adults and children aged 12 years and older with epileptic seizures. In a new early benefit ... Monday August 18, 2014 - sciencedaily.com Perampanel Demonstrates Safety, Efficacy in Treatment of Children and Adolescents With Epilepsy - Perampanel, a novel antiepileptic drug, was associated with a reduction in seizure frequency of at least 50% in more than half of children and adolescents in a meta-analysis. Perampanel was shown to ... DCGI approves Akums perampanel oral suspension for treatment of epilepsy - This approval allows perampanel oral suspension to serve as adjunctive therapy for the treatment of partial-onset seizures (POS) with or without secondarily generalised seizures, as well as primary ... High Retention, Seizure-Freedom Rates for Perampanel in Epilepsy - NASHVILLE, Tennessee ―The retention rate for the antiseizure drug perampanel (Eisai Co, Ltd) is more than 70% for adult patients with epilepsy, new research shows. In early results from the PERPRISE ... U.S. FDA Provides Response to Perampanel New Drug Application - WOODCLIFF LAKE, N.J.--(BUSINESS WIRE)--Eisai Inc. announced today that the U.S. Food and Drug Administration (FDA) has issued a Refusal to File letter in response to the company's New Drug Application ... Antiseizure Medication Perampanel Is Effective, Safe in Pediatric Patients With Epilepsy - Perampanel was found effective and safe for prolonged use in children with epilepsy, according to one study. After its 2012 approval as an antiseizure medication, perampanel (Fycompa) has been found ... Perampanel's Role in Epilepsy Care - On October 22, 2012, the US Food and Drug Administration (FDA) approved a new drug called perampanel for use in patients aged 12 years and older with partial-onset seizures. Medscape contributor ... Eisai's New Drug Application for Perampanel Designated for Priority Review by China National Medical Products Administration - For Adjunctive Treatment of Partial Onset Seizures TOKYO, Jan 4, 2019 - (JCN Newswire) - Eisai Co., Ltd. announced that its in-house discovered and developed antiepileptic drug (AED) perampanel ... Perampanel—new promise for refractory epilepsy? - Three recent phase III trials have shown that adjunctive treatment with perampanel—a first-in-class, noncompetitive AMPA antagonist—decreases seizure frequency in patients with refractory focal ... Perampanel for epilepsy: Still no proof of added benefit - The drug perampanel (trade name Fycompa) has been approved since July 2012 as adjunctive ("add-on") therapy for adults and children aged 12 years and older with epileptic fits (seizures). In a new ... Perampanel for epilepsy: still no proof of added benefit - The drug perampanel (trade name Fycompa) has been approved since July 2012 as adjunctive ("add-on") therapy for adults and children aged 12 years and older with epileptic fits (seizures). In a new ...
| ||
| Prescription | Link to this page |









