[ Home ] [ Controlled Substances ] [ Opioids ]
MPPP
|
The drug was first illicitly synthesised by a graduate student called Barry Kidston. Kidston had apparently studied a 1947 paper by Albert Zeiring. By reversing the ester of the meperidine skeleton, a drug approaching the potency of morphine was produced. However, the intermediate tertiary alcohol is liable to dehydration in acidic conditions if the reaction temperature rises above -30 degrees C, and since Kidston did not realise this and esterified the intermediate with propanoic anhydride at room temperature, MPTP was formed as a major impurity. 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPP+), a metabolite of MPTP, causes rapid onset of irreversible symptoms similar to Parkinson's Disease. MPTP is metabolized to the neurotoxin MPP+ by the enzyme MAO-B, which is expressed in neurons. This selectively kills brain tissue in the area of the brain called the substantia nigra and causes Parkinsonian symptoms.
The story began in 1947, when Demerol was discovered at Hoffman-La-Roche. At that time, drug companies were searching for pain-killing drugs that did not have the baggage of standard opioid drugs, such as hydrocodone, and oxycodone. In fact, desmethylprodine (MPPP), which is considered to be the first designer drug, was also discovered at Roche around that time, but, unlike Demerol, it was never approved.
The chemical structures of
Demerol (L) and Desmethylprodine (R)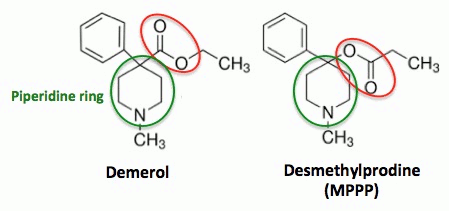
Even people with little or no chemistry knowledge can see that these two molecules are nearly identical in structure. The only difference is a functional group (red circle) that is found on the piperidine ring (green circle). In Demerol, the carbonyl group (carbon double bond to oxygen) is attached to the piperidine ring. But in MPPP, an isomer of Demerol, the carbonyl group and oxygen atom are switched, so that oxygen is attached to the piperidine. While this may seem like a trivial difference, chemically it is anything but - something that Barry Kidston, a chemistry grad student, would find out the hard way in 1976.

https://www.bluelight.org/xf/search/758545/?q=Barry+Kidston&o=date
Any organic chemist will tell you that the the bond shown in green (below) is just dying to break, and it doesn't take much to give it its wish. A little extra heat and that bond breaks, and through a process called elimination, the propyloxy group (orange circle) departs and is replaced by a carbon-carbon double bond (blue circle). Then, instead of MPPP, you have MPTP.
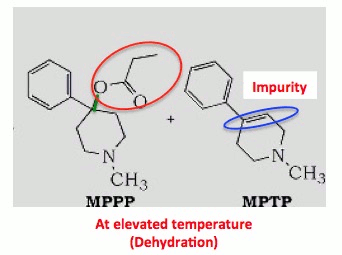
MPTP impurity formed at higher temperatures
If you're asking yourself "what's the harm in having a bit of an impurity in there?" the answer is "usually not much." But in this particular case, the impurity did something strange and unexpected with help from the brain, which is why this story is so unique.Any experienced medicinal chemist will look at the structure of MPTP and know that it is very likely to have the properties that will allow it to cross the blood-brain barrier, which it does. This is when the troubles begin. MPTP itself isn't particularly toxic, but it gets metabolized in the brain to MPP+, which is something that you do not want in your brain. Unfortunately for Kidston, he was the lab rat who would inadvertently provide the science world with a fascinating, but deadly lesson in neurochemistry.
A ubiquitous enzyme called monoamine oxidase (MAO) is responsible for the formulation and metabolism of multiple neurochemicals. It plays a crucial role in regulation of the central nervous system. In this case, the MAO in the brain "saw" MPTP and didn't "like" it, so it oxidized it to MPP+. Assuming that enzymes can make "mistakes," this is one a doozie because MPP+ is seriously bad news. It is now known that it has a particular fondness for the cells in the region of the brain called the substantia nigra. And once it gets there, all hell breaks loose. MPP+ specifically kills the dopaminergic neurons in the substantia nigra, where dopamine is normally made. The absence of dopamine is the hallmark of Parkinson's.
The bad batch of MPPP that Kidston injected into himself resulted in the development of strange symptoms within a few days. He experienced bradykinesia - a severe slowing of movement. It became so bad that Kidston was admitted to a hospital where he was diagnosed with Parkinson's Disease, which is very rare in young people. Nonetheless, Kidston did get Parkinson's - from a single injection of an impure drug.
Doctors tried a variety of neuroleptic drugs with no success, and then used L-dopa, which was first tried on Parkinson's patients (with mixed success) in the 1960s. It worked on Kidston, at least for a while. L-dopa loses effectiveness with time. This happened to Kidston, who became severely depressed and died of a cocaine overdose 18 months later.
The link between MPTP and Kidston's Parkinson's Disease was determined by analysis of the residue of drug that remained on the glassware he used. The impurities were isolated, identified and tested. Kidston has unknowingly written a chapter on the neurochemistry of Parkinson's Disease at his own expense.
Chemist Barry Kidston, who in the 1970s synthesized MPPP using Dr Ziering's recipe and a home chemistry set. However, a few days after injecting himself with a sample from a newly synthesized batch of drug, Kidston became frozen, unable to speak or walk. He was taken to the hospital by his parents, misdiagnosed with catatonic schizophrenia, and treated with electroconvulsive therapy for months. He was ultimately diagnosed him with Parkinson's disease, and improved with L-dopa treatment. Soon after, researchers analyzed the tainted drugs, and concluded that it was comprised of both MPPP and a similar compound, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Kidston died of cocaine overdose shortly afterwards. His autopsy showed loss of dopaminergic cells in the substantia nigra, the hallmark of Parkinson's disease.
The MPP+ story did not end with Kidston. In California in 1982, a cluster of six drug users who had taken "China White" all came down with Parkinson-like symptoms. When the drug was analyzed, there it was - MPTP, which was a result of a drug dealer trying to do organic synthesis in his garage. The reason for the term "frozen addicts" could not be more clear:

https://www.acsh.org/news/2017/01/12/frozen-addicts-garage-drugs-and-funky-brain-chemistry-10728

https://www.cdc.gov/mmwr/preview/mmwrhtml/00000360.htm

https://drugs-forum.com/threads/desmethylprodine.128295/

https://www.reddit.com/r/ObscureDrugs/comments/3aetdc/desmethylprodine_or_mppp/
Desmethylprodine is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche. It is an analog of pethidine (meperidine). Chemically, it is a reversed ester of pethidine which has about 70% of the potency of morphine. They found that it produced effects similar to morphine when administered to rats. They had been searching for synthetic painkillers that were less addictive than morphine. The new drug was a slight variant of pethidine. It was found to be no more effective than pethidine and was never marketed. In 1976, a 23-year-old graduate student in chemistry named Barry Kidston was searching for a way to make a legal recreational drug. Having read the paper by Ziering and Lee, he deduced that he could make a drug with pethidine's effects without its legal restrictions, since desmethylprodine is a different molecule and had never been addressed by law. Kidston successfully synthesized and used desmethylprodine for several months, after which he suddenly came down with the symptoms of Parkinson's disease and was hospitalized. It was later found that his development of Parkinson's was due to a common impurity in the synthesis of MPPP called MPTP.
Looking Ahead: The Medicare Prescription Payment Plan In 2026 - The Medicare Payment Plan lets you cut up your Part D drug payments and spread them through the entire year. 2025 has been a busy year as some of the Inflation Reduction Act initiatives have taken ... Thursday August 07, 2025 - forbes.com Prepare For Medicare’s First ‘Installment Payment Plan’: The Part D MPPP - Forbes contributors publish independent expert analyses and insights. Diane Omdahl is a Medicare expert who keeps her readers in the know. Here’s how cancer patients on Medicare can save money on treatments - Medicare Part D reforms under the Inflation Reduction Act now cap annual out-of-pocket drug costs at $2,000, offering significant relief from previous expenses that could exceed $10,000 for cancer ... Spread Medicare drug costs over 12 months-no interest charges - It's no secret that prescriptions are expensive. Between the pills, injectables and liquids, the costs can quickly add up! What a lot of people on Medicare don't know is there are several payment ... Specialty Drug Users to Gain Relief Under Medicare Reforms - The Inflation Reduction Act introduces provisions to reduce Medicare Part D out-of-pocket costs, including a $2000 annual maximum and negotiated drug prices. Significant cost reductions for etanercept ...
| ||
| Opioids | Link to this page |