[ Home ] [ Controlled Substances ] [ Opioids ]
SUFENTANIL
|
Currently sufentanil is the most potent opioid painkiller available for use in humans. Although more potent narcotic pain medications do exist, all medications stronger than sufentanil are approved for veterinary use only. It has been used intravenously and in epidurals since the 1980s but pharmaceutical company AcelRx has developed a sublingual tablet form of the drug called Dsuvia (2018)
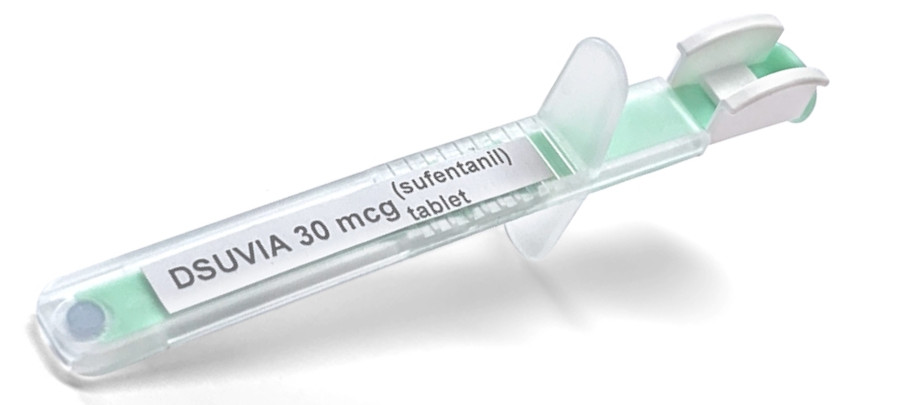
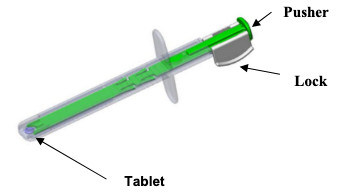
DSUVIA Single Dose Applicator
(2018) The Food and Drug Administration has approved a controversial new form of the powerful synthetic opiate sufentanil for managing acute pain in adults, just weeks after the chairman of the advisory committee warned that doing so would lead to "diversion, abuse and death." Sufentanil has been used intravenously since the 1980s, but pharmaceutical company AcelRx has developed a sublingual tablet form of the drug called Dsuvia, which is delivered through a "pre-filled, single-dose applicator." Ten times stronger than fentanyl and 500 to 1,000 times stronger than morphine, Dsuvia will be restricted to limited use only in health care settings, such as hospitals, surgery centers and emergency rooms, and will not be available in pharmacies or for home-use; nevertheless, critics fear the drug will contribute to an already devastating opioid crisis, including more than 40,000 overdose deaths last year. On October 12th, the FDA's advisory committee evaluated Dsuvia and voted 10-3 in favor of approving the drug; after the agency issued their final approval on Friday, November 2nd, Dr. Scott Gottlieb, the FDA commissioner, released a statement defending the decision and emphasizing the measures being taken to limit its use and "help prevent misuse and abuse."
Dr. Raeford Brown, the chairman of the FDA's Anesthetic and Analgesic Drug Products Advisory Committee (AADPAC), was not able to attend the meeting and vote in October due to a prior commitment, but he urged the agency's top officials to reject Dsuvia in a strongly worded letter co-signed by leaders of the consumer advocacy group Public Citizen. Brown expressed strong doubts that the agency has the capability to "regulate [Dsuvia] so that it is used only in closely controlled settings" and "only as described in the label." According to Brown, historically, the FDA has not demonstrated an ability to enforce such controls with any other opioid, and "there is currently no educational nor regulatory scheme" in place that guarantees the agency's approach to regulating Dsuvia will be any different.

https://www.rollingstone.com/culture/culture-news/opioid-fda-dsuvia-sufentanil-approved-752252/
More Potent Than Fentanyl
Sufentanil is a synthetic opioid analgesic that is five to 10 times more potent than its analogue, fentanyl (multiple brands) and 1000 times more potent than morphine. It's currently marketed for intravenous and epidural anesthesia and analgesia.
Dsuvia:
The sublingual formulation of sufentanil is designed for rapid pain relief. Its onset of action is as little as 15 minutes, and it provides about 3 hours of analgesia. Dsuvia contains 30 ug sufentanil. It comes in single-dose, prefilled applicator for sublingual administration by healthcare professionals in hospitals, surgical centers, and emergency departments. 'The single-strength tablet and single-unit packaging are designed to mitigate the possibility of dosing errors, misuse and diversion,' the company said in a news release.
2018:
The US Food and Drug Administration (FDA) has approved sufentanil sublingual tablets (Dsuvia, AcelRx Pharmaceuticals) for management of severe acute pain in adults in certified, medically supervised healthcare settings.
https://www.medscape.com/viewarticle/904330
WASHINGTON, D.C. 2018
The U.S. Food and Drug Administration (FDA) is recklessly and needlessly endangering people by approving a super-strong opioid, Public Citizen and the head of a key FDA advisory committee said today. The FDA gave the green light for the medication, which is called sufentanil sublingual tablet (brand name Dsuvia) and is to be used to treat moderate-to-severe acute pain in a medically supervised setting.
'I am very disappointed with the decision of the agency to approve Dsuvia,' Brown said. 'This action is inconsistent with the charter of the agency. As I discussed with representatives of the agency today, the lack of efficacy data and the sponsor's inadequate response to safety concerns have not been addressed since the FDA's complete response letter was sent in 2017. Clearly the issue of the safety of the public is not important to the commissioner, despite his attempts to obfuscate and misdirect. I will continue to hold the agency accountable for their response to the worst public health problem since the 1918 influenza epidemic.'

https://www.citizen.org/news/fda-makes-wrong-call-super-strong-opioid-medication-will-be-abused-and-kill-people/
(2018) FDA Statement:
In this particular case, Dsuvia is a sublingual (under the tongue) formulation of sufentanil that's delivered through a disposable, pre-filled, single-dose applicator. The medicine is restricted to use in certified medically-supervised health care settings ‒ such as hospitals, surgical centers and emergency departments ‒ for administration by a health care professional. Dsuvia, which was previously approved by the European Medicines Agency in July under the brand name Dzuveo, has some unique features in that the drug is delivered in a stable form that makes it ideally suited for certain special circumstances where patients may not be able to swallow oral medication, and where access to intravenous pain relief is not possible. This includes potential uses on the battlefield. For this reason, the Department of Defense (DoD) worked closely with the sponsor on the development of this new medicine. This opioid formulation, along with Dsuvia's unique delivery device, was a priority medical product for the Pentagon because it fills a specific and important, but limited, unmet medical need in treating our nation's soldiers on the battlefield. The involvement and needs of the DoD in treating soldiers on the battlefield were discussed by the advisory committee.

https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-approval-dsuvia-and-fdas-future-consideration


https://www.fda.gov/media/118092/download
Sufentanil is a strong opioid analgesic currently marketed for IV and epidural anesthesia. There has been published evidence that sufentanil has an analgesic effect, however, its use has been limited due to its short duration of action when delivered intravenously. The pharmaceutical attributes of sufentanil, including lipid solubility and ionization, result in rapid cell membrane penetration and onset of action. In addition, its pharmacokinetic profile when delivered sublingually could potentially avoid the high peak plasma levels and short duration of action of IV administration. In clinical trials, Sufentanil has been shown to be 5- to 10-fold more potent than fentanyl and has the following additional differences that have been observed:
- No Active Metabolites
- Rapid Transmucosal Uptake
- High Therapeutic Index
- Sufentanil is a synthetic opioid analgesic.
- Sufentanil interacts predominately with the opioid mu-receptor. These mu-binding sites are discretely distributed in the human brain, spinal cord, and other tissues.
- In clinical settings, sufentanil exerts its principal pharmacologic effects on the central nervous system.
- Its primary actions of therapeutic value are analgesia and sedation.
- Sufentanil may increase the patient's tolerance for pain and decrease the perception of suffering, although the presence of the pain itself may still be recognized.
- In addition to analgesia, alterations in mood, euphoria and dysphoria, and drowsiness commonly occur.
- Sufentanil depresses the respiratory centers, depresses the cough reflex, and constricts the pupils.
- Sufentanil's analgesic activity is, most likely, due to its conversion to morphine.
- Opioids open calcium-dependent inwardly rectifying potassium channels (OP1 receptor agonist). This results in hyperpolarization and reduced neuronal excitability.
- Sufentanil is used as an analgesic adjunct in anesthesia and as a primary anesthetic drug in procedures requiring assisted ventilation and in the relief of pain.
 Sufentanil:
Sufentanil:
https://drugs.ncats.io/drug/AFE2YW0IIZ
When given as an injection, it is administered via intravenous or epidural injection. It can be used in conjunction with anesthesia to help women going through labor. It can also be used in some cases to help with anesthesia and pain during major operations. Sufentanil is also available as a sublingual tablet sold under the brand name Dsuvia and used for acute severe pain in healthcare settings.
Doctors may choose to give a person sufentanil when other, less potent painkillers are not strong enough to relieve pain. This can occur if a person has an extreme opioid dependency. When people who are heavily opioid-dependent need surgery, standard doses of opioids may not be strong enough to overcome their high drug tolerance and relieve pain.
Side Effects:
Sufentanil may produce side effects for some people. However, these can vary depending on how the drug is given. The most common side effect with the injectable dosage form is itchy skin. Conversely, the most common side effects with the oral version of the drug are nausea, vomiting, headache, dizziness and low blood pressure.
Overdose Risk:
Sufentanil doses are carefully titrated according to the unique needs of each person. If too much is given, the result can be a dangerously low respiratory drive.
Sufentanil acts directly on the brainstem, suppressing a person's innate urge to breathe. The brainstem regulates breathing by reacting to carbon dioxide levels in the blood. When carbon dioxide levels become too high, the brainstem signals for the lungs to breathe. In the event of sufentanil overdose, this mechanism can be disrupted.
The main symptoms of an opioid overdose include slowed breathing, pinpoint pupils and significantly decreased levels of consciousness. Because the drug is limited to use in hospitals, if you suspect a person is overdosing on sufentanil, you should obtain help from a medical professional right away. They will be able to assess the person and give an opioid reversal drug like naloxone if needed.

https://www.therecoveryvillage.com/sufentanil-addiction/
This substance is extraordinarily potent (i.e. active in the microgram range), to the point that the pure powder can result in a fatal overdose if spilled on one's skin.


https://psychonautwiki.org/wiki/Sufentanil
Interactions:
Drug Interactions (442) Alcohol/Food Interactions (1) Disease Interactions (13)
What other drugs will affect Sufentanil?
A total of 442 drugs are known to interact with Sufentanil.
- 124 major drug interactions
- 302 moderate drug interactions
- 16 minor drug interactions
 Sufentanil Interactions:
Sufentanil Interactions:
https://www.drugs.com/drug-interactions/sufentanil.html
Pediatric:
Appropriate studies have not been performed on the relationship of age to the effects of sufentanil sublingual tablet in the pediatric population.Safety and efficacy have not been established.
Geriatric:
Appropriate studies performed to date have not demonstrated geriatric-specific problems that would limit the usefulness of sufentanil sublingual tablet in the elderly. However, elderly patients are more likely to have confusion and severe drowsiness and age-related liver, kidney, heart, or lung problems, which may require caution and an adjustment in the dose for patients receiving sufentanil sublingual tablet.Other Interactions:
- Grapefruit Juice
Other Medical Problems:
The presence of other medical problems may affect the use of this medicine. Make sure you tell your doctor if you have any other medical problems, especially:
- Addison's disease (adrenal gland problem)
- Alcohol abuse
- Brain tumor, history of
- Breathing or lung problems (eg, apnea, COPD, low oxygen levels, sleep apnea)
- Cor pulmonale (serious heart condition)
- Depression, history of
- Drug dependence, especially with narcotics, or history of
- Electrolyte imbalance
- Gallbladder disease
- Head injury, history of
- Heart rhythm problems (eg, slow heartbeat)
- Mental health problems, history of
- Stomach or bowel problems
- Weakened immune system - Use with caution. May increase risk for more serious side effects
- Asthma, acute or severe
- Lung or breathing problems (eg, respiratory depression), severe
- Stomach or bowel blockage (eg, paralytic ileus), known or suspected - Should not be used in patients with these conditions
- Hypotension (low blood pressure)
- Pancreatitis (swelling of the pancreas)
- Seizures, history of - Use with caution. May make these conditions worse
- Kidney disease, severe
- Liver disease - Use with caution. The effects may be increased because of slower removal of the medicine from the body
 Sufentanil (Sublingual Route) (Dsuvia):
Sufentanil (Sublingual Route) (Dsuvia):
https://www.mayoclinic.org/drugs-supplements/sufentanil-sublingual-route/description/drg-20452295
Breastfeeding:Summary of Use During Lactation:
When used epidurally or intravenously during labor or for a short time immediately postpartum, amounts of sufentanil ingested by the neonate are small and would not be expected to cause any adverse effects in breastfed infants. Labor pain medication may delay the onset of lactation; however, it appears that with good breastfeeding support, epidural sufentanil plus a local anesthetic has little or no effect on breastfeeding success.Because of sufentanil's long half-life during continued intravenous infusion or repeated intravenous administration, sufentanil levels in milk would be expected to increase if used for an extended period postpartum.
Once the mother's milk comes in, it is best to provide pain control with a nonnarcotic analgesic and limit maternal intake of sufentanil to a few days. Because there is no published experience with repeated doses of intravenous or sublingual sufentanil during established lactation, other agents may be preferred, especially while nursing a newborn or preterm infant.If the baby shows signs of increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness, a physician should be contacted immediately.
Effects in Breastfed Infants:
Newborns of breastfeeding mothers who received epidural sufentanil before and after cesarean section delivery were reportedly not clinically affected and had no differences in behavior or clinical signs over 3 days postpartum compared to newborns of mothers who received epidural sufentanil prior to delivery only.Effects on Lactation and Breastmilk:
Narcotics can increase serum prolactin. However, the prolactin level in a mother with established lactation may not affect her ability to breastfeed.Alternate Drugs to Consider:
- Acetaminophen
- Butorphanol
- Fentanyl
- Hydromorphone
- Ibuprofen
- Morphine
 Sufentanil Drug Levels and Effects:
Sufentanil Drug Levels and Effects:
https://www.ncbi.nlm.nih.gov/books/NBK501254/
| Black Box Warnings: |
|---|

Sufentanil (Rx): https://reference.medscape.com/drug/sufenta-sufentanil-343325 |
|
Therapy exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death; assess each patient's risk prior to prescribing therapy, and monitor all patients regularly for the development of these behaviors and conditions |


https://www.ema.europa.eu/en/documents/product-information/dzuveo-epar-product-information_en.pdf


https://www.dsuvia.com/pdf/FINAL_DSUVIA_Prescribing_Information_10.30.19_rev_C.pdf

https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8b580f3d-e3b5-4086-b093-87a980631147

https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=52fc28b9-464a-4173-9d0d-000ed0dbf265
Currently sufentanil is the most potent opioid painkiller available for use in humans. Although more potent narcotic pain medications do exist, all medications stronger than sufentanil are approved for veterinary use only.
- Sufentanil first was synthesized in 1974
- A synthetic opioid analgesic drug
- Approximately 5 to 10 times as potent as its parent drug, fentanyl, and 500 times as potent as morphine.
- Available as a transdermal patch similar to Duragesic in Europe under trade names such as Chronogesic
- Available as a sublingual tablet under the trade name Dsuvia
- It is essential for the administering medical professional to be trained in airway management with readily available airway equipment because the drug causes significant respiratory depression and may cause respiratory arrest if given too rapidly or in too high a dose.
Because of its extremely high potency, it is often used in surgery and post-operative pain management for patients that are heavily opioid dependent/opioid tolerant because of long term opiate use for chronic pain or illicit opiate use.
Sufentanil Citrate Injection - Sufentanil citrate 50mcg/mL; soln for IV or epidural inj. Sufentanil is an opioid agonist and is relatively selective for the mu-opioid receptor, although it can bind to other opioid receptors at ... Sunday July 28, 2024 - empr.com As Opioid Crisis Worsens, a Drug Stronger Than Fentanyl Nears FDA Approval - As America’s opioid crisis worsens, a powerful new opioid painkiller is poised to receive government approval. The Federal Drug Administration is considering a New Drug Application for a new form of ... Sublingual Sufentanil: A Solution in Search of a Problem? - For treatment of moderate-to-severe acute pain in hospital, sublingual sufentanil (Dsuvia, AcelRx Pharmaceuticals) is well-tolerated, and most side effects are mild to moderate in severity, a new ... United States DSUVIA (sufentanil) Drug Insight and Market Forecasts, 2019-2022 and 2023-2032 - ResearchAndMarkets.com - DUBLIN--(BUSINESS WIRE)--The "United States DSUVIA Drug Insight and Market Forecast - 2032" drug pipelines has been added to ResearchAndMarkets.com's offering. This report provides comprehensive ... Sufentanil Citrate Injection - Indications, Dosage, Side Effects and Precautions - Discover comprehensive details about Sufentanil Citrate Injection, including its pronunciation, uses, dosage instructions, indications, and guidelines on how and when to take it or avoid it. The ... FDA Goes Ahead With Approval of Sufentanil Despite Controversy - The US Food and Drug Administration (FDA) has approved sufentanil sublingual tablets (Dsuvia, AcelRx Pharmaceuticals) for management of severe acute pain in adults in certified, medically supervised ... Compulsive-Like Sufentanil Vapor Self-Administration in Rats - Opioid misuse is at historically high levels in the United States, with inhalation (ie, smoking and vaping) being one of the most common routes of consumption. We developed and validated a novel ... DSUVIA (sufentanil) Drug Insights and U.S. Market Analysis, 2023-2032 - Dublin, Jan. 30, 2024 (GLOBE NEWSWIRE) -- The "United States DSUVIA Drug Insight and Market Forecast - 2032" report has been added to ResearchAndMarkets.com's offering. This extensive report offers a ... Pharmacist warns of experimenting with sufentanil after drugs stolen from Saskatoon home - Julia Bareham has some hard advice for any drug user thinking about injecting the potent painkiller sufentanil: Don't do it alone, and keep a naloxone kit handy. The pharmacist with the prescription ...
| ||
| Opioids | Link to this page |