[ Home ] [ Controlled Substances ] [ Stimulants ]
PEMOLINE
|
In late October, FDA announced that pemoline will no longer be available in the United States because the risks of liver toxicity associated with the drug's use outweigh the benefits of the therapy.
Pemoline, an older therapy for attention-deficit/hyperactivity disorder (ADHD), was sold by Abbott Laboratories under the brand name Cylert until May 2005, when the company discontinued the product. According to FDA's announcement, manufacturers of generic versions of the drug have now agreed to stop marketing their formulations of pemoline in this country.
FDA, through its MedWatch drug safety reporting system, stated that pemoline products will remain available until existing stocks held by wholesalers and pharmacies are exhausted. Clinicians who prescribed pemoline for patients with ADHD must select an alternate agent, the agency stated.

https://www.formularywatch.com/view/pemoline-removed-us-market
March 2005 Petition to Remove Pemoline:
Public Citizen, representing more than 150,000 consumers nationwide, hereby petitions the Food and Drug Administration (FDA) pursuant to the Federal Food, Drug and Cosmetic Act 21 U.S.C. Section 355(e)(3), and 21 C.F.R. 10.30, to immediately remove from the market pemoline (CYLERT-Abbott Laboratories, and all generic versions), a stimulant drug for the treatment of attention deficit hyperactivity disorder (ADHD). In addition to having no demonstrated unique therapeutic benefit over other ADHD drugs such as methylphenidate, pemoline is known to have caused at least 21 cases of liver failure, including 13 resulting in liver transplantation or death. The drug's unfavorable risk to benefit ratio has led to its withdrawal in the United Kingdom and Canada, while the FDA instead opted for two separate changes to the pemoline label, in 1996 and 1999. A 2002 FDA study has clearly demonstrated that these labeling changes failed to increase monitoring for liver toxicity or ensure only second-line use of pemoline.[1] In light of this evidence of unique liver toxicity without evidence of unique therapeutic benefit, we contend that the only responsible course of action is to remove this dangerous drug from the market.

https://www.citizen.org/article/petition-to-remove-the-attention-deficit-drug-pemoline-cylert-from-the-market/
NEJM Comment:
The FDA should be applauded for removing pemoline from the list of approved medications for children with ADHD. The risk for severe hepatotoxicity was established several years ago, and the boxed warning in 1999 was a limited response, although it did reduce the prescription rate. Pediatricians who treat children with ADHD can choose from three other medications (2 stimulants and a nonstimulant), although none are without potential side effects. The pemoline story reminds us to be cautious about long-term medication use and to remain informed about adverse effects that surface after drug approval.

https://www.jwatch.org/pa200512090000003/2005/12/09/fda-alert-pemoline-market-withdrawal
June 1999 ADHD Association: Drug Deaths Are Preventable:
WASHINGTON, June 28 /U.S. Newswire/ - The makers of Cylert (pemoline) cautioned that this product "should not ordinarily be considered as first line drug therapy for ADHD." The warning came as the result of the drug's association with a higher risk of life-threatening hepatic (liver) failure and a dozen or more deaths. Patients must have a normal baseline liver function test, and liver function tests are suggested to be done every two weeks. A consent form must be signed prior to use of this drug. "It took 15 cases of liver failure and some deaths to start warning parents," said Lynn Murphy, executive director of the Feingold Association, a non-profit organization assisting parents who have children with the disorder. "Children died from a drug to help them get along in school," continued Murphy.
The National Institutes of Health estimates ADHD affects between 3 to 5 percent of school age children in this country.

https://www.newswise.com/articles/pemoline-not-first-line-drug-for-adhd
Pemoline Information:
![]()
Pemoline was a prescription medication (at least in the USA), marketed under the names of Cylert, Betanamin, Tradon, and Ceractiv. Considered to be a central nervous system stimulant, it was previously used to help treat children with ADHD. It was useful for increasing attention and decreasing restlessness in children who are easily distracted and have trouble concentrating for longer periods of time. Pemoline was first synthesized back in 1913, but not much regarding the activity or its action of mechanism was known for several decades after its discovery.
The drug is currently classified as an oxazoline substance, which simply means a compound containing one atom of oxygen and nitrogen which help to protect carboxylic acids.
In addition to the primary use as a treatment for childhood ADHD, Pemoline has also been prescribed for treating narcolepsy. It is currently classified as a psychotropic and Schedule IV drug (non-narcotic type) within the US. Pemoline is now considered to be unsafe by many experts and is no longer prescribed for ADHD treatment. The mechanisms of action for Pemoline are still not fully understood, despite the length of time it has been available for study. It is, of course, considered to be a powerful and effective central nervous system stimulant.

https://nootriment.com/pemoline/
Pemoline is a central nervous system stimulant that derives at least some of its effects by increasing levels of dopamine in the brain.
Dopamine is one of several neurotransmitters in the brain. Neurotransmitters are naturally occurring chemicals that regulate the transmission of nerve impulses from one cell to another. Mental and physical well-being are partially dependent on maintaining the proper balance among the various neurotransmitters in the brain.
Pemoline is similar in its effects to dextroamphetamine and methylphenidate, two other drugs used to treat ADHD, although it is not chemically related to these drugs.The mechanism of action of CNS stimulants in the treatment of ADHD is not totally clear, but probably includes increased mental alertness, decreased mental fatigue, and an increased sense of well-being.
Pemoline should not be used as a substitute for psychological, educational, and social support in treating people with ADHD. Because pemoline may be associated with liver toxicity (poisoning causing liver damage), it should be used only after trying other drugs to treat ADHD. Patients should try dextroamphetamine or methylphenidate first.
Pemoline is a central nervous system stimulant. Pemoline is structurally dissimilar to the amphetamines and methylphenidate. Pemoline is generally considered dopaminergic, but its precise method of action hasn't yet been definitively determined. The interaction of pemoline with other drugs has not been studied in humans. The following are adverse reactions in decreasing order of severity within each category associated with pemoline: hepatic dysfunction, aplastic anemia, convulsive seizures, hallucinations, insomnia, anorexia and weight loss.
 Pemoline:
Pemoline:
https://drugs.ncats.io/drug/7GAQ2332NK
(Pemoline (Cylert) is no longer available in the U.S.)
Medication Treatments for ADHD:
Pemoline (Cylert) for ADHD. Cylert ranks third in sales for the treatment of ADHD. Cylert is manufactured by Abbott; no generic is available. Unlike other stimulant medications Cylert has an onset of action of about an hour and must be taken for 1-2 weeks before improvement occurs. It is recommended that the dosage of this medication be increased in increments of 18.75mg every 2-3 days over several weeks. Cylert is more expensive than Ritalin or Dexedrine.

https://www.healthyplace.com/adhd/articles/medication-treatments-for-adhd-pemoline-cylert-for-adhd
APA Dictionary Pemoline:
A nonamphetamine CNS stimulant used for the management of attention-deficit/hyperactivity disorder. Its effects resemble those of the amphetamines and methylphenidate, and its mechanism of action includes blockade of dopamine reuptake. Pemoline has been associated with rare but occasionally fatal liver failure and with the development of Tourette's disorder. Safety concerns led to its withdrawal from the Canadian market in 1999 and the U.S. market in 2005. Former U.S. trade name (among others): Cylert.

https://dictionary.apa.org/pemoline
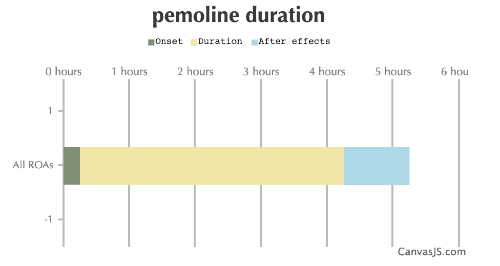
| Duration: A stimulant of the 4-oxazolidinone class. Was used as a medication for ADHD and Narcolepsy, yet was pulled from most markets due to liver failures among children. | |||
| Route | Onset | Duration | After Effects |
|---|---|---|---|
| Tripsit Factsheets | |||

Pemoline Basic Information: http://drugs.tripsit.me/pemoline | |||
| All ROAs: | 15-30 minutes | 4-6 hours | 1-6 hours |
| |||
Side Effects:
Common side effects of Cylert (pemoline) include:
- Insomnia (Difficulty Sleeping)
- Nervousness
- Headache
- Drowsiness
- Mild Depression
- Nausea
- Abdominal Discomfort
- Diarrhea
- Decreased Appetite (Anorexia)
- Weight Loss
- Rapid Heart Rate
- Rash
https://www.rxlist.com/cylert-drug.htm
Pediatric:
Slowed growth rate in children who received medicines like pemoline for a long period of time has been reported.Some doctors recommend medicine-free periods during treatment with pemoline to help prevent slowed growth.
Pemoline may make behavior worse in children with serious mental illness.Other Medical Problems:
Make sure you tell your doctor if you have any other medical problems, especially:
- Drug abuse or dependence (or history of) - Dependence on pemoline may develop
- Gilles de la Tourette's syndrome or other tics
- Liver disease
- Mental illness (severe) - Pemoline may make the condition worse
- Kidney disease - Higher blood levels of pemoline may occur, increasing the chance of side effects
 Pemoline (Oral Route) (Cylert):
Pemoline (Oral Route) (Cylert):
https://www.mayoclinic.org/drugs-supplements/pemoline-oral-route/description/drg-20068359
What is the dosage for Cylert (pemoline)?
Cylert (pemoline) is administered as a single oral dose each morning. The recommended starting dose is 37.5 mg/day. This daily dose should be gradually increased by 18.75 mg at one week intervals until the desired clinical response is obtained. The effective daily dose for most patients will range from 56.25 to 75 mg. The maximum recommended daily dose of pemoline is 112.5 mg.
Clinical improvement with Cylert (pemoline) is gradual. Using the recommended schedule of dosage titration, significant benefit may not be evident until the third or fourth week of drug administration.
Where possible, drug administration should be interrupted occasionally to determine if there is a recurrence of behavioral symptoms sufficient to require continued therapy.

https://www.medicinenet.com/pemoline/article.htm
Special Precautions:
Should only be started in patients with normal baseline LFTs; monitor LFTs every 2 wk. Discontinue if serum alanine aminotransferase is increased, if signs of liver failure develop or if no substantial clinical response within 3 wk of completing dose titration. Renal dysfunction, psychosis, bipolar disorder, DM, cardiovascular disease, seizure disorders, insomnia, porphyria, or hypertension. Potential for drug dependency. Avoid abrupt withdrawal in chronic patients. May impair ability to drive or operate machinery. Pregnancy and lactation.

https://www.mims.com/malaysia/drug/info/pemoline?mtype=generic
Interactions:
Drug Interactions (87) Alcohol/Food Interactions (2) Disease Interactions (13)
What other drugs will affect Pemoline?
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. When you are taking pemoline, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive. Using pemoline with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Iobenguane I 131
A total of 87 drugs are known to interact with Pemoline.
- 14 major drug interactions
- 72 moderate drug interactions
- 1 minor drug interaction
 Pemoline (Oral) Interactions:
Pemoline (Oral) Interactions:
https://www.drugs.com/cons/pemoline.html
Because of its association with life threatening hepatic failure, CYLERT should not ordinarily be considered as first line drug therapy for ADHD (see INDICATIONS AND USAGE). Because CYLERT provides an observable symptomatic benefit, patients who fail to show substantial clinical benefit within 3 weeks of completing dose titration, should be withdrawn from CYLERT therapy.


https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/016832s022_017703s018lbl.pdf
Synonyms:
Pheniminooxazolidinone, Phenoxazole, Phenylisohydantoin, Phenylpseudohydantoin, Azoksodon, Azoxodon, Azoxodone, Betanamin, Centramin, Constimol, Cylert, Cylert Chewable, Dantromin, Deltamin, Deltamine, Endolin, Fenoxazol, Fio, Hyton, Hyton asa, Juston-Wirkstoff, Kethamed, Myamin, NPL 1, Nitan, Notair, Okodon, PIO, Pemolin, Pemolina, Phenalone, Phenilone, Pioxol, Pomoline, Pondex, Ronyl, Senior, Sigmadyn, Sistra, Sistral, Stimul, Stimulol, Tradon, Tradone, Volital, Volitol, Yh 1, Bebia Ointment Pommade (Magnesium Pemoline + Magnesium Silicate + Zinc Oxide)
A Reddit Thread:
Let's talk pemoline
- I wanna hear some experience reports about it. But, I'll start the discussion.
- It's well known for it's hepatotoxic effect. It was in fact withdrawn due to this. it was in use for approximately 30years and enjoyed being among the most highly prescribed adhd medication available. Then those pesky problems showed their heads. Some 20people experienced severe, mostly fatal liver problems. Out of millions that had taken it over the years. It also still is in use as basically the only adhd med in Japan. It sounds pretty safe when the scope of it's use is added to that statistic
- It's reported to be between caffeine and amphetamine, and slightly stronger than methylphenidate. Not bad, especially after you read point 3
- The synthesis seems to be very easy. The precursors could be produced from otc and uncontrollable ingredients. I'm Not getting into that here, because...they could be purchased cheaply and without scrutiny. These are not suspicious. This means it's a simple 2step synthesis
- This is technically an extension of 3, but
- Step 1 methyl mandelate is produced by refluxing Mandelic acid and methanol with concentrated sulfuric acid. Yield is almost quantitative
- Step 2 guanidine nitrate is added to aaa methanolic solution of sodium(hydroxide)* too which methyl mandelate is then added and allowed to reflux. * guanidine hydrochloride can be used as well, or freebase guanidine in which case the alkali need Not be used
- I don't know about roa. According to wiki it is often snorted or injected. It is acidic rather than alkaline as most other drugs, meaning it can not form a salt with acids, ie no freebase, no hydrochloride,etc. It is(was) available as a magnesium salt(is that the right word? I think so
- I've used it before, and it is a fairly pleasant stimulant. It's great for functional tasks (work, studying, etc.) but not so great for recreational use because it lacks any real degree of euphoria, (although it does give a pleasant mood lift) and it is probably even worse for your liver at above therapeutic dosages. I can't find my notes on it from when I took it, but Heres what I do remember: My ideal dosage range was 20-50mg of the magnesium chelate orally. It kicks in in about 20 minutes taken on a mostly empty stomach (4 hrs after eating) and has a very gentle onset with few cardiovascular effects unlike amphetamine and methylphenidate. The effects plateau after about an hour and a half and last for a solid six or eight hours. The comedown is not bad, (much gentler than Adderall) although when I did not eat after taking it my resting heart rate speed up considerably, but I blame this more on low blood sugar because it has happened on other stimulants. With sublingual administration, the effects are more pronounced but last less than half the time compared to oral and has a shittier comedown.
Some other comments about it: it doesn't do much in terms of the pupils and insufflation doesn't work terribly well compared to oral and sublingual ROA's. It is a fairly strong appetite suppressant and this effect lasts for a few hours after the stimulant effects are gone, so be sure to eat to keep your blood sugars from crashing. The weirdest thing I noticed is that pemoline is much more prone (at least with me) to causing "hyper focus" than other stimulants, probably because tasks seem uniquely gratifying on pemoline in a way that they don't on other stims.

https://www.reddit.com/r/TheeHive/comments/9mttvl/lets_talk_pemoline/
Pemoline Search Results:
https://www.reddit.com/search/?q=pemoline

https://drugs-forum.com/threads/pemoline-magnesium-for-super-brains.2413/

http://www.inchem.org/documents/pims/pharm/pim940.htm
A stimulant drug. It was first synthesized in 1913 but its activity was not discovered until the 1930s. It was used as a medication to treat attention-deficit hyperactivity disorder (ADHD) and narcolepsy. It is no longer generally available in the United States. Pemoline passes the blood - brain barrier and acts as a surrogate for dopamine, not affecting endogenous intracellular dopamine.There is some data to suggest that pemoline is a nootropic acting as a catalyst conductor in the synapses of the brain's memory centers, raising the efficiency of memory and assisting RNA formation in the brain.
In some patients pemoline is suspected of causing hepatotoxicity. Since receiving FDA approval in 1975, it has been linked with 21 cases of liver failure, of which 13 resulted in liver replacement or death. Over 200,000 children with ADHD were prescribed pemoline in the US and Canada alone during the 25 years it was available so the number of liver failure cases was statistically not that large. However the reactions proved idiosyncratic and unpredictable, with patients sometimes taking the drug with no issue for months or even years, before suddenly developing severe liver toxicity. There was no clear exposure-toxicity relationship, and no characteristic liver pathology findings. Some patients showed as little as one week between first appearance of jaundice and complete liver failure, and some of the patients that developed liver failure had not showed elevated liver transaminase levels when tested previously.
Pemoline - Indications, Dosage, Side Effects and Precautions - Learn everything you need to know about Pemoline-pronunciation, uses, dosage guidelines, indications, and when to take or avoid it. Get up-to-date information on side effects, precautions, warnings, ... Thursday November 07, 2024 - medindia.net Side effect(s) of Pemoline - Review the side-effects of Pemoline as documented in medical literature. The term "side effects" refers to unintended effects that can occur as a result of taking the medication. In majority of the ...
| ||
| Stimulants | Link to this page |







