[ Home ] [ Controlled Substances ] [ Stimulants ]
SIBUTRAMINE
|
Discontinued in many countries
Subitramine is a potent inhibitor of monoamines (serotonin, dopamine, noradrenaline) reuptake that was approved by FDA for the treatmen of obesity. Sibutramine is metabolized to metabolites M1 and M2 which are more active toward the monoamine transporters.The drug was withdrawn from the market because of clinical trial data indicating an increased risk of heart attack and stroke. It was sold under a variety of brand names including Reductil, Meridia and Sibutrex.
 Subitramine:
Subitramine:
https://drugs.ncats.io/drug/WV5EC51866
Sibutramine (trade name Meridia in the USA, Reductil in Europe and other countries), usually as sibutramide hydrochloride monohydrate, is an orally administered agent for the treatment of obesity.
- It is a centrally acting stimulant chemically related to amphetamines.
- Sibutramine is classified as a Schedule IV controlled substance in the United States.
- In October 2010, Sibutramine was withdrawn from Canadian and U.S. markets due to concerns that the drug increases the risk of heart attack and stroke in patients with a history of heart disease.
- Belongs to the class of organic compounds known as chlorobenzenes.
 Metabocard for Sibutramine:
Metabocard for Sibutramine:
https://hmdb.ca/metabolites/HMDB0015237
1998 Article:
Sibutramine hydrochloride monohydrate, a schedule IV controlled substance that is structurally related to amphetamine, has been approved by the U.S. Food and Drug Administration for the treatment of obesity. Medical Letter consultants reviewed current information about this new drug.
Sibutramine inhibits the reuptake of norepinephrine, serotonin and dopamine. Through this mechanism, concentrations of neurotransmitters are increased in the brain. The drug is rapidly absorbed from the gastrointestinal tract and is almost completely metabolized on its first pass through the liver. The active metabolites reach a peak concentration in serum in three to four hours, are primarily excreted in urine and partly in feces, and have a half-life of 14 to 16 hours.

https://www.aafp.org/afp/1998/0801/p542.html
Emerging Drug List 2001:
Sibutramine is a beta-phenylethylamine that exhibits monoamine reuptake-inhibitor activity for norepinephrine, serotonin, and to a lesser extent, dopamine. The precise mechanism by which sibutramine acts as an anorexiant is not known. However, sibutramine is believed to act by decreasing food/energy intake or increasing energy expenditure, or a combination of both. The recommended starting dose is 10 mg daily. If, after four weeks, the desired effects are not achieved, the dose can be increased to 15 mg daily. Patients who do not tolerate the 10 mg dose can have the dose reduced to 5 mg daily. Meridia is available in capsule form in two strengths (i.e., 10 mg, and 15 mg).


https://www.cadth.ca/media/pdf/108_No3_sibutramine_edrug_e.pdf
February 1, 2009 - Question:
What is Meridia (sibutramine), how does it work, and how effective is it in reducing weight?Answer:
Meridia is a brand name for a product known as sibutramine. It's considered to be an appetite suppressant. It's available only by prescription and it is approved for longer-term use, unlike some of the older appetite-suppressing medications. Meridia works by boosting the levels of two chemical messengers in the brain: norepinepherine and serotonin.And amplifying this signal from these chemical messengers triggers the satiety center - the satisfaction center in the brain - and helps us feel satisfied earlier in eating episodes that we can effectively have had enough to eat before we've consumed quite as much food as we might otherwise without the medication.
(This) translates into relatively modest additional weight loss, perhaps another 3-5 pounds beyond what we might accomplish if we were simply following a diet and exercise plan. And it may help us achieve a little bit more success in keeping the weight off over the long term.
 (2009 Article) What Is Meridia (Sibutramine), How Does It Work, And How Effective Is It In Reducing Weight?
(2009 Article) What Is Meridia (Sibutramine), How Does It Work, And How Effective Is It In Reducing Weight?
https://abcnews.go.com/Health/WellnessResource/story?id=6770136
Sibutramine Oral User Reviews:
24 Total User Reviews Sibutramine Oral Read Reviews Condition: Overweight (22 Reviews): Effectiveness (4.36) Ease of Use (4.64) Satisfaction (4.14)
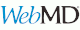 User Reviews & Ratings - Sibutramine Oral:
User Reviews & Ratings - Sibutramine Oral:
https://www.webmd.com/drugs/drugreview-5405-sibutramine

https://www.drugs.com/comments/sibutramine/for-obesity.html
Liver:
In large clinical trials, sibutramine therapy was not associated with serum enzyme elevations, and it has only rarely been implicated in cases of clinically apparent, acute liver injury.
Sibutramine Hepatotoxicity:
Sibutramine has not been linked to an increased rate of serum enzyme elevations during therapy, but the results of serum ALT monitoring have been reported only rarely. Despite its long term availability, only a single case report of acute liver injury attributed to sibutramine has been published. The time to onset was 2 weeks and the pattern of liver enzyme elevation was cholestatic. The liver injury was anicteric and self-limited in course (Case 1). Immunoallergic and autoimmune features were absent. There have been no reports of acute liver failure or chronic liver injury attributed to sibutramine.
 Sibutramine Overview:
Sibutramine Overview:
https://www.ncbi.nlm.nih.gov/books/NBK548248/
Breastfeeding:Summary of Use During Lactation:
Sibutramine is no longer marketed in the United States.Because there is no published experience with sibutramine during breastfeeding, an alternate therapy may be preferred, especially while nursing a newborn or preterm infant.
Information on the effect of sibutramine on serum prolactin is somewhat conflicting.
 Sibutramine Drug Levels and Effects:
Sibutramine Drug Levels and Effects:
https://www.ncbi.nlm.nih.gov/books/NBK500562/
(2002) Petition to Ban Sibutramine:
Public Citizen, a nationwide consumer organization, with a membership of more than 130,000, petitions the FDA, pursuant to the Federal Food, Drug and Cosmetic Act 21 U.S.C. Section 355(e)(3), and 21 C.F.R. 10.30, to immediately ban the unacceptably dangerous prescription diet drug Meridia (sibutramine, Knoll Pharmaceuticals/Abbott). According to the FDA data base, since its launch in early 1998 sibutramine has now been associated with 29 deaths including 19 from cardiovascular adverse effects in people using this minimally effective drug.

https://www.citizen.org/article/petition-to-ban-the-diet-drug-sibutramine-meridia/
Side Effects Warning:
Sibutramine substantially increases blood pressure and/or pulse rate in some patients. Regular monitoring of blood pressure and pulse rate is required when prescribing sibutramine.
In placebo-controlled obesity studies, sibutramine 5 to 20 mg once daily was associated with mean increases in systolic and diastolic blood pressure of approximately 1 to 3 mm Hg relative to placebo, and with mean increases in pulse rate relative to placebo of approximately 4 to 5 beats per minute. Larger increases were seen in some patients, particularly when therapy with sibutramine was initiated at the higher doses.Blood pressure and pulse should be measured prior to starting therapy with sibutramine and should be monitored at regular intervals thereafter. For patients who experience a sustained increase in blood pressure or pulse rate while receiving sibutramine, either dose reduction or discontinuation should be considered. MERIDIA should be given with caution to those patients with a history of hypertension, and should not be given to patients with uncontrolled or poorly controlled hypertension.

https://www.medicinenet.com/sibutramine/article.htm
Safety Announcement:
[10-8-2010] The U.S. Food and Drug Administration (FDA) is recommending against continued prescribing and use of Meridia (sibutramine) because this drug may pose unnecessary cardiovascular risks to patients. FDA has requested that Abbott Laboratories - the manufacturer of Meridia - voluntarily withdraw this drug product from the United States market. Abbott has agreed to voluntarily stop marketing of Meridia in the United States.

https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-recommends-against-continued-use-meridia-sibutramine
European Commission final decision:
The European Medicines Agency has completed a review of the safety and effectiveness of sibutramine. The Agency's Committee for Medicinal Products for Human Use (CHMP) has concluded that the benefits of sibutramine do not outweigh its risks, and that all marketing authorisations for medicines containing sibutramine should be suspended throughout Europe.
The European Commission issued a decision on 6 August 2010.

https://www.ema.europa.eu/en/medicines/human/referrals/sibutramine


https://www.ema.europa.eu/en/documents/referral/opinion-following-article-31-referral-sibutramine-international-non-proprietary-name-inn-sibutramine_en.pdf

https://medsafe.govt.nz/profs/PUArticles/WithdrawalofSibutramine.htm

https://www.tga.gov.au/alert/sibutramine-reductil-withdrawal-australia
https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2010/14614a-eng.php

https://www.hsa.gov.sg/announcements/press-release/hsa-suspends-sales-of-sibutramine-products-with-immediate-effect

https://www.nejm.org/doi/full/10.1056/nejmoa1003114
(2010 Article) What about those without Cardiovascualar issues?
The licence for the anti-obesity drug sibutramine has been suspended in Europe: a decision that casts considerable doubt on the ability of the European Medicines Agency (EMA) to correctly apply scientific principles. Preliminary, unpublished data from the Sibutramine Cardiovascular Outcomes (SCOUT) trial have revealed a possible significant increase in cardiovascular events, in a group of individuals for whom the drug has always been contraindicated: those with cardiovascular disease(CVD). The assumption has been made by the EMA that everyone with a BMI of greater than or equal to 27 must have CVD, meaning that nobody in Europe is allowed to take sibutramine, despite a decade of use in real life practice, and a full portfolio of clinical trials, which revealed no danger signals. The concept of primary versus secondary prevention has been arrogantly disregarded by the Agency. Although the possible rise in cardiovascular events in this high-risk group is troubling, and suggests that the known sporadic increases in blood pressure and pulse rate may be more sinister than was previously thought, the evidence profile for the use of sibutramine in obese individuals without CVD is still strongly favourable.


https://web.archive.org/web/20150726041800/http://www.practicaldiabetes.com/SpringboardWebApp/userfiles/espdi/file/Ldr%20Haslam_Layout%201.pdf


https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020632s034s035lbl.pdf

https://www.medschat.com/Discuss/Sibutramine/

https://drugs-forum.com/tags/sibutramine/

https://www.reddit.com/search/?q=sibutramine


https://www.caymanchem.com/msdss/14476m.pdf
Discontinued in many countries
- An appetite suppressant
- Originally developed in 1988
- Was widely marketed and prescribed until 2010
In 2010 the FDA noted the concerns that sibutramine increases the risk of heart attacks and strokes in patients with a history of cardiovascular disease
- Has been associated with increased cardiovascular events and strokes
- Has been withdrawn from the market in several countries and regions
- In October 2011, the FDA warned that 20 brands of dietary supplements were tainted with sibutramine.
Obesity drug sibutramine (Meridia): hypertension and cardiac arrhythmias - Reason for posting: Sibutramine has been taken off the market in Italy after 50 adverse events (primarily tachycardia, hypertension and arrhythmias) and 2 deaths from cardiovascular causes were ... Monday May 13, 2002 - cmaj.ca FDA Advisors Say Sibutramine's CV Risks Warrant Harsher Restrictions or Withdrawal - September 15, 2010 (Updated September 16, 2010) (Adelphi, Maryland) — An FDA advisory panel has voted to recommend that the FDA either severely restrict access to the weight-loss drug sibutramine ... Abbott Laboratories Voluntarily Withdraws Weight-loss Drug Sibutramine (Meridia®) from the Canadian Market - OTTAWA - Health Canada is informing healthcare practitioners and Canadians that Abbott Laboratories is voluntarily withdrawing the prescription weight-loss drug sibutramine, which is marketed under ... Abbott Withdraws Sibutramine From Market - October 8, 2010 — Abbott Laboratories has withdrawn the obesity drug sibutramine (Meridia) from the market in light of clinical trial data pointing to an increased risk for stroke and myocardial ... Australia Bans Obesity Drug Sibutramine - Australia has banned obesity drug sibutramine after a study showed it could cause a fatal heart attack or stroke. It has already been withdrawn from the US market. Australia has banned obesity drug ... The effect of sibutramine prescribing in routine clinical practice on cardiovascular outcomes: a cohort study in the United Kingdom - The marketing authorization for the weight loss drug sibutramine was suspended in 2010 following a major trial that showed increased rates of non-fatal myocardial infarction and cerebrovascular events ... Hydro Pineapple Burn dietary supplement recalled due to undeclared sibutramine - The FDA has released the following: Houston, Texas, eBay Seller ID: jongu 4308 is voluntarily recalling all lots of Hydro Pineapple Burn to consumer level. FDA analysis has found the product to ... No compelling evidence that sibutramine prolongs life in rodents despite providing a dose-dependent reduction in body weight - The health and longevity effects of body weight reduction resulting from exercise and caloric restriction in rodents are well known, but less is known about whether similar effects occur with weight ... Sibutramine combination Price - Details concerning the Sibutramine combination medication encompass its pricing as well as its availability in various forms, including tablets, capsules, syrups, creams, gels, ointments, liquids, and ... Supplements Pulled for Undeclared Sibutramine - FDA tests prompted recall by marketer of 'natural' weight-loss supplements. Feb. 5, 2010— -- The marketer of three "natural" dietary weight-loss supplements issued a voluntary recall of the ... Top obesity drug sibutramine being suspended - A leading obesity drug is being suspended from use in the UK amid fears it increases the risk of heart attacks and strokes. The Medicines and Healthcare Products Regulatory Agency has told doctors to ... Top obesity drug sibutramine being suspended - A leading obesity drug is being suspended from use in the UK amid fears it increases the risk of heart attacks and strokes. The Medicines and Healthcare Products Regulatory Agency has told doctors to ...
| ||
| Stimulants | Link to this page |