[ Home ] [ Controlled Substances ] [ Opioids ]
NICOMORPHINE
|
Nicomorphine is the 3,6-dinicotinate ester of morphine. It is a strong opioid agonist analgesic two to three times as potent as morphine. The 3,6-diesters of morphine are drugs with more rapid and complete central nervous system penetration due to increased lipid solubility and other structural considerations. Whilst this produces an enhanced "bang" when the drug is administered intravenously, it cannot be distinguished from morphine via other routes, although the different side effect profile, including lower incidence of nausea, is very apparent.



Nicomorphine [Mass] of Dose:
https://loinc.org/loinc/4348-9/
https://loinc.org/loinc/4348-9/
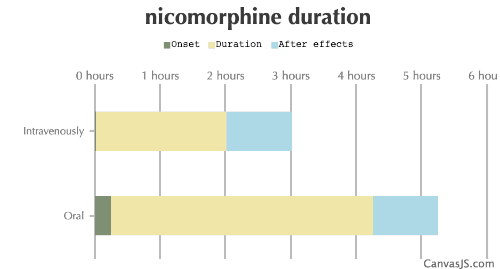
| Duration: Opioid agonist that is 2-3x the potency of Morphine. Is used in few countries. Rapid onset of effects, due to the increased lipid solubility. | |||
| Route | Onset | Duration | After Effects |
|---|---|---|---|
| Tripsit Factsheets | |||

Nicomorphine Basic Information: http://drugs.tripsit.me/nicomorphine | |||
| Oral: | 15-30 minutes | 4-6 hours | 1-6 hours |
| Intravenously: | 1-2 minutes | 2-4 hours | 1-6 hours |
| |||
| Avoid: All other CNS depressants. | |||
| Rapid onset of effects, due to the increased lipid solubility. | |||
Doses:
Nicomorphine hydrochloride is an opioid analgesic used in the treatment of moderate to severe pain. It is given in oral doses of 5 to 10 mg daily or by intramuscular, slow intravenous, or subcutaneous injection in doses of 10 to 20 mg; higher doses have also been used. It may also be given rectally in usual doses of 10 to 20 mg daily.

Nicomorphine hydrochloride Profile:
https://drugster.org/analgesics-anti-inflammatory-drugs-and-antipyretics/nicomorphine-hydrochloride
https://drugster.org/analgesics-anti-inflammatory-drugs-and-antipyretics/nicomorphine-hydrochloride
Nicomorphine was first synthesized in 1904 and was patented as Vilan by Lannacher Heilmittel G.m.b.H. of Austria in 1957. It is used, particularly in the German-speaking countries and elsewhere in Central Europe and some other countries in Europe and the former USSR in particular, for post-operative, cancer, chronic non-malignant and other neuropathic pain. It is commonly used in patient-controlled analgesia (PCA) units. Nicomorphine's side effects are similar to those of other opioids and include itching, nausea and respiratory depression. It is considered by doctors to be one of the better analgesics for the comprehensive mitigation of suffering, as opposed to purely clouding the noxious pain stimulus, in the alleviation of chronic pain conditions. Nicomorphine is regulated in much the same fashion as morphine worldwide but is a Schedule I controlled substance in the United States and was never introduced there. Nicomorphine may appear on rare occasions on the European black market and other channels for unsupervised opioid users. It can be produced as part of a mixture of salts and derivatives of morphine by end users by means of treating morphine with nicotinic anhydride or related chemicals in an analogue of the heroin homebake process.

| Opioids | Link to this page |




