[ Home ] [ Controlled Substances ] [ Opioids ]
HYDROMORPHONE
|

Hydromorphone
Hydromorphone (also known as dihydromorphinone and the brand name Dilaudid among others) is a more potent opioid analgesic than morphine and is used for moderate to severe pain. It can be administered by injection, by infusion, by mouth, and rectally. Oral bioavailability is low. The kidney excretes hydromorphone and its metabolites. Some metabolites may have greater analgesic activity than hydromorphone itself but are unlikely to contribute to the pharmacological activity of hydromorphone. With the exception of pruritus, sedation and nausea and vomiting, which may occur less after hydromorphone than after morphine, the side-effects of these drugs are similar. Hydromorphone interacts predominantly with the opioid mu-receptors. These mu-binding sites are discretely distributed in the human brain, with high densities in the posterior amygdala, hypothalamus, thalamus, nucleus caudatus, putamen, and certain cortical areas. It also binds with kappa and delta receptors which are thought to mediate spinal analgesia, miosis and sedation.
 Hydromorphone Hydrochloride:
Hydromorphone Hydrochloride:
https://drugs.ncats.io/drug/L960UP2KRW
Dilaudid was the brand name of the first drug containing hydromorphone. It was introduced in 1926 and remained the only brand name drug that contained hydromorphone for more than 50 years. Today, people commonly refer to generic versions of hydromorphone as Dilaudid. These medications come in pill, injection and liquid forms. Hydromorphone is a prescription painkiller that belongs to a class of drugs called opioids. It's two to eight times stronger than morphine. For decades, Dilaudid was a popular alternative to morphine for the treatment of pain. Other brand names for drugs containing hydromorphone include Exalgo, introduced in 1984, and Palladone, introduced in 2004. Both drugs came in extended-release pill formulations. Palladone was withdrawn from the market in 2005 because it caused potentially fatal side effects when mixed with alcohol.

https://www.drugrehab.com/addiction/prescription-drugs/hydromorphone/
Hydromorphone is a potent schedule II opioid analgesic drug. Hydromorphone abuse has been a continuing problem in the United States. It is marketed as injectable ampoules, multiple dose vials, tablets and suppositories. Hydromorphone is indicated for relief of moderate-to-severe pain. Hydromorphone is marketed under brand names, Dilaudid and Exalgo. It is also marketed in generic forms. Street Names: Dust, Juice, Smack, D, Footballs.
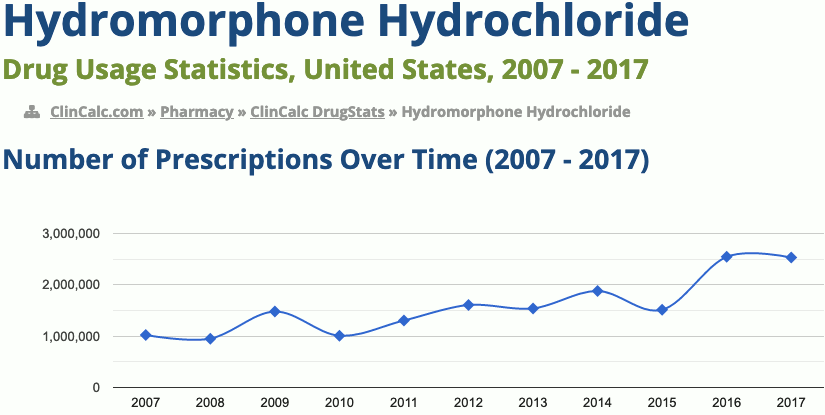
205th most prescribed medicine in the United States for 2017

https://clincalc.com/DrugStats/Drugs/HydromorphoneHydrochloride
Hydromorphone belongs to the group of medicines called narcotic analgesics (pain medicines). It acts on the central nervous system (CNS) to relieve pain. When a narcotic medicine is used for a long time, it may become habit-forming, causing mental or physical dependence. However, people who have continuing pain should not let the fear of dependence keep them from using narcotics to relieve their pain. Mental dependence (addiction) is not likely to occur when narcotics are used for this purpose. Physical dependence may lead to withdrawal side effects if treatment is stopped suddenly. However, severe withdrawal side effects can usually be prevented by gradually reducing the dose over a period of time before treatment is stopped completely.
This medicine is available only under a restricted distribution program called the Opioid Analgesic REMS (Risk Evaluation and Mitigation Strategy) program.
 Hydromorphone (Oral Route) (Dilaudid | Dilaudid-5 | Exalgo | Palladone):
Hydromorphone (Oral Route) (Dilaudid | Dilaudid-5 | Exalgo | Palladone):
https://www.mayoclinic.org/drugs-supplements/hydromorphone-oral-route/description/drg-20074171
Pediatric:
Appropriate studies have not been performed on the relationship of age to the effects of hydromorphone injection in the pediatric population.Safety and efficacy have not been established.
Geriatric:
Appropriate studies performed to date have not demonstrated geriatric-specific problems that would limit the usefulness of hydromorphone injection in the elderly. However, elderly patients are more likely to have age-related lung, kidney, liver, or heart problems, which may require caution and an adjustment in the dose for patients receiving hydromorphone injection in order to avoid serious side effects.Other Interactions:
- Ethanol
Other Medical Problems:
Make sure you tell your doctor if you have any other medical problems, especially:
- Addison disease (adrenal gland problem)
- Alcohol abuse, acute
- Breathing or lung problems, severe (eg, asthma, apnea, low oxygen levels)
- Chronic obstructive pulmonary disease (COPD)
- Cor pulmonale (serious heart condition)
- Depression, history of
- Drug dependence, especially narcotic abuse or dependence, or history of
- Gallbladder disease or gallstones
- Head injury, history of
- Hypothyroidism (an underactive thyroid)
- Kyphoscoliosis (curvature of the spine with breathing problems)
- Mental health problems, history of
- Problems with passing urine
- Prostatic hypertrophy (enlarged prostate, BPH) - Use with caution. May increase risk for more serious side effects
- Allergy to sulfites
- Stomach or bowel blockage (eg, paralytic ileus) - Should not be used in patients with these conditions
- Hypotension (low blood pressure)
- Pancreatitis (inflammation of the pancreas)
- Seizures, history of - Use with caution. May make these conditions worse
- Kidney disease
- Liver disease - Use with caution. The effects may be increased because of slower removal of the medicine from the body
- Patients who are not opioid-tolerant - Dilaudid-HP should not be given in these patients
 Hydromorphone (Injection Route):
Hydromorphone (Injection Route):
https://www.mayoclinic.org/drugs-supplements/hydromorphone-injection-route/description/drg-20074244
Dilaudid is one of the more powerful synthetic narcotics in the opioid class of drugs and an addiction to Dilaudid can rapidly develop through continued use. People regularly taking Dilaudid build up a tolerance to the drug, requiring larger and more frequent doses to get the desired effects. Users can develop a tolerance to Dilaudid within two or three weeks. Once a tolerance takes hold, users taking the pills more frequently often finish their prescription ahead of schedule. This is due to the regular dose no longer working the same way on the body as they were prescribed to do, because the body is used to taking them. This can result in physical dependence or even addiction even if the user is taking the medication as prescribed.
Doctors prescribe Dilaudid for pain related to cancer and serious injuries, such as burns. The time it takes for Dilaudid to take takes effect varies by how it is taken. When taken orally, Dilaudid typically takes effect within 30 minutes to an hour. When used intranasally, it typically takes 5 minutes, and its effects are almost immediate when taken intravenously. Regardless of the method of administration, the pain-relieving effects of Dilaudid typically last between four and six hours. The drug attaches to receptors in the brain and central nervous system to dull pain. Dilaudid also triggers the release of excessive amounts of dopamine in the brain, causing pleasurable feelings. This activates the reward center of the brain, which interprets the event as something that is important and should be repeated. The more this happens, the less the brain will naturally produce dopamine, and the more reliant the body becomes on Dilaudid.
Though I'd never taken them before I got sober, I was prescribed Vicodin and was given Dilaudid at the hospital and those drugs felt utterly wonderful coursing through my veins. But therein lies the rub; you feel too good when you take them.
Those who abuse Dilaudid recreationally may mix it with alcohol and/or benzodiazepines to get a better high. All three of these drugs are central nervous system depressants. Mixing these drugs amplifies their effects but also dangerously slows breathing and heart rates. Mixing Dilaudid with these drugs can lead to respiratory failure, coma, seizure, or even a fatal overdose. People addicted to Dilaudid often want to relive the euphoric and relaxed feelings they initially experience with the drug, so they continue trying to replicate this "rush." This often leads to abusing harder drugs, like heroin, which are often more accessible.

https://www.addictioncenter.com/opiates/dilaudid/
Hydromorphone (trade names Palladone IR, Palladone SR, Dilaudid and numerous others) is a potent centrally-acting analgesic drug of the opioid class; it is a derivative of morphine, specifically a hydrogenated ketone thereof - therefore a semi-synthetic drug and both an opiate and a true narcotic.
https://www.patientslikeme.com/treatment/hydromorphone
Hydromorphone belongs to a class of drugs called "opioids," which includes morphine. It has an analgesic potency of two to eight times greater than that of morphine and has a rapid onset of action.
It is legally manufactured and distributed in the United States.
hydromorphone is taken to produce feelings of euphoria, relaxation, sedation, and reduced anxiety. It may also cause mental clouding, changes in mood, nervousness, and restlessness. It works centrally (in the brain) to reduce pain and suppress cough.
Hydromorphone use is associated with both physiological and psychological dependence.


https://www.getsmartaboutdrugs.gov/sites/getsmartaboutdrugs.com/files/files/Hydromorphone_R.pdf
Here is an estimated range of times, or detection windows, during which hydromorphone can be detected by various testing methods:
Detection Windows
- Urine: 3 to 4 days
- Blood: Up to 24 hours
- Saliva: Up to 2 days
- Hair: Up to 90 days
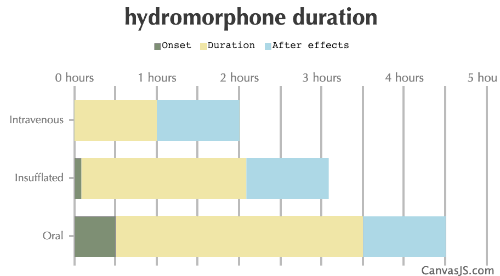
| Duration: An opioid that is roughly 5x the potency of IV Morphine. Only comes in IR tablets in the US, and is given mostly as XR in other countries. It is frequently used in hospitals for short, but immediate pain relief during procedures requiring the patient to be awake. Also frequently used as a recreational opiate. | |||
| Route | Onset | Duration | After Effects |
|---|---|---|---|
| Tripsit Factsheets | |||

Hydromorphone Basic Information: http://drugs.tripsit.me/hydromorphone | |||
| Intravenous: | 0-1 minutes | 1-2 hours | 1-6 hours |
| Insufflated: | 5-10 minutes | 2-3 hours | 1-2 hours |
| Oral: | 30-60 minutes | 3-4 hours | 1-6 hours |
| |||
| Avoid: All other CNS depressants. | |||
| Effects: Euphoria, Dry Mouth, Mood lift, Itchiness, Relaxant, Constipation, Pupil Constriction, Analgesia. | |||
https://www.webmd.com/drugs/2/drug-1423/hydromorphone-injection/details
Important Information:
MISUSE OF OPIOID MEDICINE CAN CAUSE ADDICTION, OVERDOSE, OR DEATH. Keep the medication in a place where others cannot get to it.
Taking opioid medicine during pregnancy may cause life-threatening withdrawal symptoms in the newborn.
Fatal side effects can occur if you use opioid medicine with alcohol, or with other drugs that cause drowsiness or slow your breathing.
Do not drink alcohol. Dangerous side effects or death could occur.
Avoid driving or hazardous activity until you know how hydromorphone will affect you. Dizziness or drowsiness can cause falls, accidents, or severe injuries.
Interactions:
Drug Interactions (394) Alcohol/Food Interactions (1) Disease Interactions (17)
What other drugs will affect Hydromorphone?
Opioid medication can interact with many other drugs and cause dangerous side effects or death. Be sure your doctor knows if you also use:This list is not complete. Other drugs may affect hydromorphone, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible interactions are listed here.
- other narcotic medications - opioid pain medicine or prescription cough medicine
- a sedative like:
- drugs that make you sleepy or slow your breathing - a sleeping pill, muscle relaxer, medicine to treat mood disorders or mental illness
- drugs that affect serotonin levels in your body - a stimulant, or medicine for depression, Parkinson's disease, migraine headaches, serious infections, or nausea and vomiting
A total of 394 drugs are known to interact with Hydromorphone.
- 116 major drug interactions
- 277 moderate drug interactions
- 1 minor drug interactions
 Hydromorphone Drug Information:
Hydromorphone Drug Information:
https://www.drugs.com/mtm/hydromorphone.html
| Side Effects: |
| Get emergency medical help if you have signs of an allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat. |
|---|
| RxList |
Dilaudid Information: https://www.rxlist.com/dilaudid-side-effects-drug-center.htm |
| Opioid medicine can slow or stop your breathing, and death may occur. A person caring for you should seek emergency medical attention if you have slow breathing with long pauses, blue colored lips, or if you are hard to wake up. |
Call your doctor at once if you have:
|
| Seek medical attention right away if you have symptoms of serotonin syndrome, such as: agitation, hallucinations, fever, sweating, shivering, fast heart rate, muscle stiffness, twitching, loss of coordination, nausea, vomiting, or diarrhea. |
| Serious side effects may be more likely in older adults and those who are malnourished or debilitated. |
| Long-term use of opioid medication may affect fertility (ability to have children) in men or women. It is not known whether opioid effects on fertility are permanent. |
Common side effects may include:
|
| This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
| Maximum Dosage: | |
|---|---|
| Prescribers Digital Reference | |

Hydromorphone Hydrochloride - Drug Summary: https://www.pdr.net/drug-summary/Dilaudid-Injection-and-HP-Injection-hydromorphone-hydrochloride-490 | |
| Adults: | With appropriate dosage titration, there is no maximum dose of hydromorphone. |
| Geriatric: | With appropriate dosage titration, there is no maximum dose of hydromorphone. |
| Adolescents: | With appropriate dosage titration, there is no maximum dose of hydromorphone. The safety and efficacy of extended-release tablets have not been established. |
| Children: | With appropriate dosage titration, there is no maximum dose of hydromorphone. The safety and efficacy of extended-release tablets have not been established. |
| Infants: | With appropriate dosage titration, there is no maximum dose of hydromorphone. The safety and efficacy of extended-release tablets have not been established. |
| Neonates: | Safety and efficacy have not been established. |
| Black Box Warnings: |
|---|

Dilaudid, Exalgo (hydromorphone) (Rx): https://reference.medscape.com/drug/dilaudid-hydromorphone-343313 |
Opioid analgesic risk evaluation and mitigation strategy (REMS)
Hydromorphone high-potency formulation
Risk of medication errors
Addiction, abuse, and misuse
Life-threatening respiratory depression
Accidental exposure
Neonatal opioid withdrawal syndrome
Risks from concomitant use with benzodiazepines or other CNS depressants
|
Breastfeeding:Summary of Use During Lactation:
Limited data indicate that hydromorphone is excreted into breastmilk in small amounts, but large maternal dosages have caused neonatal central nervous system depression.In general, maternal use of oral narcotics during breastfeeding can cause infant drowsiness, central nervous system depression and even death.
Hydromorphone use should be limited in nursing mothers.
Newborn infants seem to be particularly sensitive to the effects of even small dosages of narcotic analgesics. Once the mother's milk comes in, it is best to provide pain control with a nonnarcotic analgesic and limit maternal intake of hydromorphone to a few days at a low dosage with close infant monitoring.If the baby shows signs of increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness, a physician should be contacted immediately.
Drug Levels:
In adults, hydromorphone has an oral bioavailability of 62% and is metabolized to inactive metabolites.
While not commonly used in infants, an appropriate dose for this age group is 10 mcg/kg parenterally or 30 mcg/kg orally every 4 hours as needed.Effects on Lactation and Breastmilk:
Narcotics can increase serum prolactin.
However, the prolactin level in a mother with established lactation may not affect her ability to breastfeed.Alternate Drugs to Consider:
- Acetaminophen
- Ibuprofen
- Morphine
 Hydromorphone Drug Levels and Effects:
Hydromorphone Drug Levels and Effects:
https://www.ncbi.nlm.nih.gov/books/NBK501227/
IMPORTANT WARNING:
Hydromorphone may be habit forming, especially with prolonged use. Take hydromorphone exactly as directed. Do not take more of it, take it more often, or take it in a different way than directed by your doctor. Hydromorphone may cause serious or life-threatening breathing problems, especially during the first 24 to 72 hours of your treatment and any time your dose is increased. Taking certain medications during your treatment with hydromorphone may increase the risk that you will develop serious or life-threatening breathing problems, sedation, or coma. Do not drink alcohol, take prescription or nonprescription medications that contain alcohol, or use street drugs during your treatment. Swallow the extended-release tablets whole. Do not split, chew, dissolve, or crush them. If you swallow broken, chewed, crushed, or dissolved tablets you may receive too much hydromorphone at once instead of receiving the medication slowly over time. This may cause serious breathing problems or death. Talk to your doctor about the risks of taking hydromorphone.
 Hydromorphone Information:
Hydromorphone Information:
https://medlineplus.gov/druginfo/meds/a682013.html
Liver:
Neither hydromorphone nor oxymorphone have been linked to serum enzyme elevations during therapy or to clinically apparent liver injury.
Hydromorphone Hepatotoxicity:
As with most opiates in current use, therapy with hydromorphone and oxymorphone has not been linked to serum enzyme elevations. There have been no convincing cases of idiosyncratic acute, clinically apparent liver injury attributed to either agent.
 Hydromorphone Overview:
Hydromorphone Overview:
https://www.ncbi.nlm.nih.gov/books/NBK548775/


https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/019892s015lbl.pdf


https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019034s018lbl.pdf


https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019891s024,019892s029lbl.pdf

https://mothertobaby.org/fact-sheets/hydromorphone/pdf/


http://akorn.com/documents/catalog/package_inserts/17478-540-50.pdf
https://www.webmd.com/drugs/drugreview-6102-hydromorphone-er.aspx?drugid=6102&drugname=hydromorphone-er

https://www.drugs.com/comments/hydromorphone/for-chronic-pain.html

https://drugs-forum.com/forums/hydromorphone.403/
Dilaudid is the purest form of synthetic Heroin available thru pharmaceutical companies. Meant strictly for patients prescribed the drug for moderate to severe pain, "D's" are also very common on the streets for having the best intravenous high available of any opiate, yes even better than actual Heroin. (In my opinion.) In pill form 4MG and 8MG rein. No "cut" no 10MG Opiate///325MG Acetametaphine bullshit

https://www.urbandictionary.com/define.php?term=dilaudid
Drugs that have similar effects include:
Heroin | Morphine | Hydrocodone | Fentanyl | Oxycodone
Hydromorphone:
Hydromorphone is made from morphine
- An opioid
- Used to treat moderate to severe pain.
- It may be used by mouth or by injection into a vein, muscle, or under the skin
- Effects generally begin within half an hour and last for up to five hours.
- Common side effects include dizziness, sleepiness, nausea, itchiness, and constipation.
- Serious side effects may include abuse, low blood pressure, seizures, respiratory depression, and serotonin syndrome.
- Hydromorphone 2 mg by mouth is equivalent to approximately 10 mg morphine by mouth.
- Patented in 1923
Typically, long-term use is only recommended for pain due to cancer.
In 2017, it was the 205th most commonly prescribed medication in the United States, with more than two million prescriptions
Virtual clinics prescribe safer-supply drugs to people as opioid crisis deepens - Three relatively new virtual health-care clinics in London are prescribing opioids to people who would otherwise buy toxic drugs on the street. Tuesday April 23, 2024 - cbc.ca ‘Drug injecting room by stealth’: Sky News host raises concerns for health centre - Sky News host Peta Credlin has questioned whether Australians can trust the Victorian Labor government on drug injecting rooms in the state. “They said before the election they’d never have a drug ... VPD deputy chief says 'there's no question' safe-supply drugs are being diverted - The deputy chief of the Vancouver Police Department told a House of Commons committee this week that 50 per cent of hydromorphone seizures in B.C. had been diverted from “safe supply” drugs. The ... Large drug bust in St. Thomas results in charges for two suspects - A large drug bust in St. Thomas has resulted in a slew of charges for two people. On April 10, Elgin-Middlesex OPP, along with St. Thomas police, searched a residence in St. Thomas. Police said they ... Adam Zivo: B.C. police are determined to ignore 'safer supply' opioids flooding streets - According to the 2018/19 British Columbia Controlled Prescription Drug Atlas (more recent data is unavailable), there were approximately 80,000 hydromorphone patients in B.C. that year. Three people face drug and weapon charges after search of Pictou County home: N.S. RCMP - The Pictou County Integrated Street Crime Enforcement Unit (PCISCEU) has charged three people with drugs and weapons offences after police searched a home in Three Brooks, N.S. Two charged after cocaine, pot, other drugs seized in raid - Two St. Thomas residents are facing a string of drug charges after a police raid at a home in the city turned up suspected cocaine and methamphetamine. Two St. Thomas residents are facing a string of ... Major Drug Bust in Constance Lake First Nation nearly $19,000 CAD in Substances Seized - Major drug bust at Constance Lake First Nation home sees one arrested, with nearly $19,000 CAD in substances seized ... Stolen BMW seized in drug bust in Caledon; Brampton and Mississauga residents charged - A BMW was seized and four people were charged in a drug trafficking bust in Caledon. A drug trafficking investigation, involving multiple regions including Kawartha Lakes, Dufferin, Muskoka, ... Feds will keep working with BC on 'science-based, compassionate' drug decriminalization project: Trudeau - Justin Trudeau has told British Columbians he wants to continue working with the province on its “science-based, compassionate” drug decri. Federal addictions minister meets with B.C. counterpart as backlash continues on decriminalization - about 20 per cent have the prescription as part of B.C.’s program of providing safer pharmaceutical alternatives to street drugs. But she said about half of the hydromorphone pills seized by ...
| ||
| Opioids | Link to this page |